
Atypical HUS: Research & Drug Development Landscape 2020
As the year 2020 continues to be shaped in terms of ‘The Year of COVID-19’, research related to the pandemic has launched collaborations and led to advancements that are likely to expand the knowledge base and landscape for rare diseases and orphan drugs. Key areas for crossover in research and treatment are COVID-19 discoveries in medical fields dealing with immunity, inflammation, complement, multi-organ involvement, and thrombotic microangiopathy. Such scientific advancements tangential to the COVID-19 pandemic may provide welcome news to those affected by one or more of the estimated 7,000 rare disorders, such as atypical HUS, and for the medical professions dedicated to providing treatment and healthcare management for rare disease patients.
Those with interests in the atypical HUS arena must simultaneously look forward and peripherally to gain the broader view of growth potential for future aHUS treatments. While we advocate for better patient outcomes within a disease-specific population that’s very rare, the aHUS Alliance is highly interested in progress made for other medical conditions where tiny clots damage organs, or those where the body’s immune system has cascaded into an over-activated response. We’re heartened by approval of a second therapeutic drug (ravulizumab) to treat atypical HUS (eculizumab was prior) and encouraged by more robust pharma drug research and pipelines in 2020. At the same time, it would be remiss not to note lack of progress in important areas such as: limited drug access in most nations, ineffective patient engagement practices, need for increased physician education, and moribund outreach throughout the clinical trials process. While perhaps unusual for advocates in the rare disease space offer a patient-centric “drug landscape report” on orphan drugs of interest to them, the aHUS Alliance provides our unique viewpoint so that patients, caregivers, policy makers, and industry leaders may understand the interests and concernss of those directly affected by the rare disease atypical hemolytic uremic syndrome (known also as atypical HUS or aHUS).
What interests us in 2020 regarding aHUS therapeutic drug R & D?
Complement: It’s Everywhere
While the first half of 2019 was fairly quiet regarding the advancements specific to atypical HUS, its last quarter and first part of 2020 made up for this with industry news and research publications of interest. The aHUS community, along with the rest of the world, learned about COVID-19 research and the treatment regarding complement-associated clotting activity in multiple organs (Magro et al. 2020). The title of this April 2020 article by Campbell and Kawash provides insight into our interest in this topic, Will Complement Inhibition Be the New Target in Treating COVID-19–Related Systemic Thrombosis? New knowledge about the complement cascade and its mechanisms will be helpful to better understand a wide variety of diseases. Atypical HUS is sometimes termed CM-aHUS (complement-mediated aHUS) or grouped together with other forms of CM-TMA (complement-mediated thrombotic microangiopathy). Aptness of terminology for CM-aHUS hits a bit of a snag with genetic mutation DGKE-caused aHUS (S Quaggin, 2013), “Notably, mutations in DGKE are not associated with activation of the complement pathway, the only other identified cause of this disorder so far, and have important implications for patient management.” As Thurmond and Quintrec remind us, “Does one need to completely inhibit the complement system? Complement activation is not a binary process. There is continual low-level activation in the plasma, and in health there is a delicate balance between activating and inhibitory factors.” While interest in complement inhibitors continues to be strong, research teams and industry have widened their net to include a broader look at new approaches, particularly as related to TMA and complement therapeutics.
Changing Partners & Strategies
Multiple pharmaceutical companies mentioned in our aHUS Alliance 2018 pharma overview have been acquired or established collaborative relationships to strengthen or deepen their research and product pipeline. Omeros Corporation announced a research collaboration alliance with the University of Cambridge, which at present is focused on research related to OMS721 targeting MASP-2, “the effector enzyme of the complement system’s lectin pathway, and to OMS906, Omeros’ lead human monoclonal antibody targeting MASP-3, the key activator of the complement system’s alternative pathway.” In January 2020 Alexion completed the acquisition of Achillion Pharmaceuticals, which due to reduced drug production expense may provide in theory a lower-cost alternative to eculizumab (and therefore an affordable drug for low resource nations) should Achillion’s oral, small molecule Factor D inhibitor (Danicopan, ACH-4471) perform well in clinical trials. Information or studies on comparison of different drugs would naturally be of high interest to the aHUS community as well. The ‘Eculizumab to Cemdisiran Switch in aHUS (DANCE)‘ clinical trial NCT03999840 ,with the drug formerly designated as ALN-CC5, will continue to be ‘One to Watch’ as this trial progresses. Needless to say, it’s exceedingly difficult to follow rare drug and clinical trial advancements when there are multiple designations or name versions for one drug, or when studies/trials utilize descriptive names. Apellis has conducted a phase III clinical trial comparing their therapeutic drug APL-2 or Pegcetacoplan to eculizumab for treating PNH. A comparison study between eculizumab and ravulizumab, both products of Alexion Pharmaceuticals, was done for the PNH population and which perhaps may be conducted with aHUS patients at some future point.
Collaborative efforts and initiatives among aHUS research teams are also of high interest to aHUS patients around the world, but stakeholders should agree in principal and action that therapeutic drug development must ensure “values of research accountability, data transparency and validation through the scientific peer-review process” (Mastellos et al, 2019). Studies on optimal duration and dosing regimens for eculizumab therapy are being conducted in multiple nations, such as SETSaHUS (UK), CUREiHUS (NL), and STOPECU (FR). The global aHUS patients’ research agenda targeted 15 key questions which aHUS families and advocacy groups feel would benefit from additional research including: predisposing genetic and triggering factors of aHUS, development of effective and low-cost therapeutic drugs, determining optimal times to diagnose and prevent development of TMA before it becomes a catastophic aHUS episode, genetic testing to guide treatment and patient care, and varied topics such as the socio-economic impacts of aHUS.
Productive Pathways: One size does not fit All
As scientists gain deeper knowledge of disease processes and pathways, we can better target new treatments and inform the drug development process. Manufacture of a synthetic drug in a laboratory yields a fairly simple chemical molecule at a lower cost and quicker production rate than biologic drugs. Biologic drugs or biopharmaceuticals are grown from living cell cultures and take time to grow within very precise conditions. (See GaBi: Small molecule versus Biologic Drugs) Biologically active human factor H, such moss-made Factor H, remains intriguing as are developments regarding action of moss-produced MFHR1 on complement activation (the alternative pathway). The concept of structure-guided drug design, or creation of a molecule that binds to a specific location of a target, may prove useful for certain patient populations such as for those who exhibit eculizumab resistence. People with atypical HUS can present with very different clinical profiles, so future disease management may require creation of aHUS patient subgroups according to genetic variants then treated with more tailored care or a ‘precision medicine’ approach.
Targeted treatment for certain medical conditions are familiar in treatment protocols for cancer patients, where genetic testing guides clinical decisions about what drug is most effective for that particular patient in order to treat malignant cells. While we’re more used to seeing genetics play a different role in aHUS research, we have become increasingly aware of its potential role in aHUS therapeutic drug discovery. Silence Therapeutics in the UK has the technology to “selectively inhibit any gene in the genome, specifically silencing the production of disease-causing proteins” and has newly teamed with AstraZeneca for ‘gene silencing drugs’ which deliver therapeutic siRNA molecules to target cells implicated in renal, cardiovascular and other diseases. The Genzyme/Sanofi product research lineup includes Lademirsen (SAR339375) for Alport Syndrome which targets miRNA-21, implicated in acute kidney injury.
Getting new aHUS treatments to market
Longer-acting therapeutic drugs and different delivery systems (oral or injection SC), as well as differences in target selection, are appearing in more articles and aHUS research. It’s a long and costly process for a new therapeutic drug to reach market, with only about 10% of drugs successfully moving from research and preclinical stages to becoming a safe, effective, and approved drug. According to biopharma industry source PhRMA on The Process Behind New Medicines, average cost of research and development (R&D) for a new drug to reach market is $2.6 billion. There is increased competition across the therapeutic drug landscape, with expansion in targeted products and in more treatments for rare diseases. Creation of drugs at a lower price point will improve access for patients but push the pharmaceutical industry to explore creative solutions in order to reduce their production times, improve flexibility of plants, and increase product output while still lowering drug costs. The field of biosimilars to eculizumab, approved to treat aHUS since 2011, has expanded in the past year. Biosimilars are drugs that replicate a known biologic drug and pose unique drug production problems, since copying living cell cultures needs to yield a replica drug that is both as effective and safe as the original. Generium’s Elizaria is the first biosimilar to eculizumab to reach market, and “The Russian branded product, Elizaria, will be about 25% cheaper than the original drug.”, as reported in a expense comparison of a 24-month eculizumab regimen costing up to $700,250 in some Western nations or $400,000-$580,000 in Russia. Other companies are currently developing biosimilars to eculizumab within their product pipelines, such as Amgen, ISU Abxis, and Samsung Bioepis with some clinical trials already launched as with ABP 959 and SB12 in clinical trial for PNH (more links in our Pharma Chart 2020).
Overcoming & Addressing Issues to Move Forward
Treatment Cost & Access
Drug cost can drive drug access, particularly for the rare disease community. No matter how life-saving or transformative a therapy may be, if it is not an affordable treatment its value is sharply limited. Stated as a collective global vision for aHUS patients, “When enough aHUS/cTMA awareness helps everyone in the world get affordable access to the right treatment they need to be cured and be normal, it will be a good day.” Atypical HUS patients, and the clinicians who treat them, still await that day. The interwoven issues of drug cost and economic burdens combine to drive national orphan drug policies, something that has not gone unnoticed by aHUS stakeholders and which has garnered more attention in the past year. Alexion’s ravulizumab (Ultomiris®) gained American FDA approval for PNH in December 2018, followed by approval as an aHUS therapeutic drug in October 2019. In June 2020, coinciding with release of this aHUS Alliance review, Alexion announced positive results on a Phase 3 study of ravulizumab SC for PNH patients, a new delivery method (self-administered shot) that should improve patients’ quality of life in treatment aspects and likely in other QoL measures as well. While little information is available to gauge treatment costs, the effect of ravulizumab’s extended dosing intervals should reduce some burdens for patients in terms of treatment frequency (8 weeks, with longer plasma residence than 2 weeks for eculizumab) and may also lessen burdens on healthcare systems. The aHUS Alliance noted with interest the topical overview outlined by Ryan et al (Dec 2019) on pharmacoeconomic issues regarding eculizumab therapy for aHUS patients. That still does not address the reality that aHUS patients in the majority of the world live in nations that do not have access to eculizumab (or ravulizumab), or that healthcare systems in the remaining minority of nations restrict eculizumab therapy by aHUS clinical subtype or limit access by allowed duration of treatment. Atypical HUS patients in low resource nations have not been able to access eculizumab at all, and proposals by clinicians to remedy this through an international aHUS drug access model have largely been ignored by Alexion. In their 2019 publication on next-generation complement therapeutics, Mastellos et al noted, “Moreover, the excessive costs of current anti-C5 therapy (typically, some US $500,000 per year and patient, depending on the market) cannot be overlooked, since they impose a sizeable economic burden on health-care systems and limit drug access in developing countries.” In that same publication the authors provide an overview of regulatory and economic aspects in Box 2, stating in part “With the socioeconomic and ethical implications becoming increasingly conspicuous, drug regulatory bodies as well as medical and patient communities are now faced with urgent decision-making to reshape orphan drug development in complement indications and beyond.” (For more on this topic, see our section titled Patients’ Perspective: Eculizumab Challenges in the medical publication Optimal management of atypical hemolytic uremic disease: challenges and solutions. Raina et al, 2019).
Moving forward: Good will Come Together
People and groups interested developments regarding the aHUS drug landscape must employ a telescope as well as a microscope to gain a more detailed look at potential for expansion of treatments available for atypical HUS patients. A key goal for medical professionals and industry will be more effective outreach to front-line clinicians, and to create or seek avenues that encourage early aHUS diagnosis protocols which also can support clinical trial enrollment. Patients and aHUS advocacy groups must work jointly with other stakeholders in the aHUS space to build more effective pathways for engagement in a wider variety of collaborations, which would allow academics and industry to better understand patient experiences and form a stronger framework for future initiatives. While we advocate for better patient outcomes within a disease-specific population that’s very rare, the aHUS Alliance is highly interested in progress made for other medical conditions where tiny clots damage organs, or those where the body’s immune response has been over-activated.
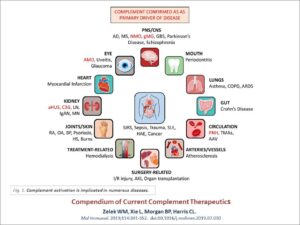
Figure 1. Complement activation is implicated in numerous diseases.
Source for above diagram: Compendium of current complement therapeutics. Zelek WM, Xie L, Morgan BP, Harris CL. Mol Immunol. 2019;114:341-352. doi:10.1016/j.molimm.2019.07.030
aHUS Alliance – Our 2020 Overview of Pharmaceutical Pipelines
aHUS Alliance – a 2020 Overview of Medical Conditions with research avenues of interest to the aHUS community
Please note that COVID-19 and associated medical conditions (MIS-C, ARDS and others) are interlinked within this article.
In Conclusion
In May 2020 an aHUS Alliance article pointed out that expert opinions on defining aHUS continue to vary greatly, listing 16 sources to find lack of agreement in clinically describing atypical HUS which remains “a syndrome in need of clarity” (Berger, 2018). Complement activation is implicated in numerous diseases (Zelek et al, 2019). Complement and thrombotic microangiopathy (TMA) appear frequently as common keywords in aHUS research, but how many physicians suspecting an atypical HUS diagnosis in an urgent care situation can screen and sort the varied terminology across nephrology, hematology, and immunology journals? Quick and accurate diagnosis of aHUS impacts many facets, to include not only patient outcomes but also clinical trial enrollment which require that patients be ‘treatment naive’ (no previous treatment for the illness or condition). Graphics for physicians such as the KDIGO Reference Guide for aHUS & C3G illustrate the need to incorporate multiple key elements, such as: pathways of the complement system, use of lab analysis such as ADAMTS13 to distinguish TTP from aHUS, and a diagnostic flowchart to differentiate thrombotic microangiopathies. Atypical HUS usually impacts kidney function, but damage to the function of other organs often results in the need for a multi-disicplinary care approach to deal with issues involving the heart, lungs, GI tract, nervous system or other areas that required specialized medical care. Newly diagnosed patients and atypical HUS family caregivers often are confused by medical terms and publications, so in addition to aHUS resources and other content created by the aHUS Alliance and within our Info Centre, we’re encouraged by more patient-friendly materials and recent expansions of international collaborations among clinicians and aHUS investigators. Connecting fragmented information flow and globally establishing broader integration of advocacy partnerships can provide a firmer foundation for future aHUS research advancements, as well as support for a robust aHUS therapeutic drug landscape.
Each year the aHUS Alliance joins with patients and their families around the world to invite and engage the researchers, clinicians, industry professionals, and the public with raising awareness about this very rare disease.
Learn more about aHUS Awareness Day, created by the aHUS Alliance as an annual 24 September campaign to highlight the issues and challenges facing the international aHUS community. Follow aHUS Awareness Day on Twitter. #aHUS24Sept #aHUSday #SHUa24Sept #aHUSaware
Connect with Us: Info@aHUSallianceAction.org
Facebook: @aHUS Alliance
Twitter: @aHUSallianceAct
Atypical HUS, our dedication to Global Action: Network & Link Connections
Int’l aHUS Advocacy: National Patient Groups
aHUS Clinicians & Investigators

L Burke
