
The aHUS Alliance recently conducted an interview with Dr Ralf Reski, a professor and researcher in the field of moss-made biopharmaceuticals. Of particular interest to us is Moss-FH, given its future potential as a therapeutic drug for patients with atypical HUS. As a founder of Greenovation Biotech, Dr Reski and a biotech research team at the Reski Lab work on a variety of efforts to include the study of gene functions and analysis of regulatory networks while developing biopharmaceuticals against human diseases made from moss plants.
We appreciate Dr Reski’s willingness to participate in the interview, which provides a unique aHUS perspective into the world of moss biopharmaceuticals. The aHUS Alliance invites others to share their research, industry perspectives, or academic work through an interview. Please contact us for questions or comments at: info@aHUSallianceAction.org
- The aHUS Alliance included Moss-FH in previous reports regarding potential therapeutic drugs for future treatment of atypical HUS. Can you tell us more about this moss-made version of human complement Factor H?
Human factor H is a difficult-to-express protein because it is so large and so complexly folded. In fact, moss is so far the only expression host to produce sufficient amounts of FH for preclinical testing. Another potential issue is that FH is a glycoprotein, which means it is decorated with specific sugar residues. We modified the moss in such a way that this decoration is very similar to what is found in humans. But we still have to do some work to obtain a fully “humanized” moss.
- Moss-FH is in preclinical stages for C3 Glomerulopathy (C3G). How does this relate to the atypical HUS patient population, and what about other genetic variants than factor H such a CFI, CD46, PLG and others?
Moss-FH is intended as enzyme-replacement therapy (ERT) for patients that are deficient in this protein. It is unlikely that it will complement other defects. Nevertheless, it may be an alternative to Eculizumab (Soliris), a monoclonal antibody with limited treatment options and severe side effects. Above that, the treatment with Soliris is very expensive.
- Medicines work in different ways. What are the mechanics, pharmacology, and drug delivery anticipated for Moss-FH as a therapeutic drug?
All results from our preclinical trials with bioassays and with FH-deficient mice tell us that moss-FH may be even better than human FH, because it is very homogenously decorated with specific sugar residues. It will need to be injected intra-venously like most other biopharmaceuticals.
- We are familiar with the phrase ‘nature’s medicine cabinet’. What’s the story of the plant-based Moss-FH as opposed to a monoclonal antibody like eculizumab or a manufactured chemical compound?
The moss Physcomitrella patens is a green plant that can be grown in pure mineral media in bioreactors. It is genetically stable and can be engineered with outstanding precision so that it very efficiently produces human glycoproteins with a superb quality. The production process does not involve supplements like antibiotics and moss does not contain pathogens that may harm humans. Therefore, the production process is safe, stable, and much cheaper than other production systems. We estimate that we will be able to produce such drugs at about 10% of the costs of other systems. Because we do not use animal products, moss-made drugs can be regarded as kosher or halal. And it is a vegan product, which may be an advantage for some of us.
- What else is important to share regarding Moss-FH or your research?
I started with moss research in 1984. Since then I have developed this system from scratch and am happy that it is now a widely used model species in biology. Because I foresaw the potential of moss for biotechnology, I founded the company Greenovation Biotechnology in 1999. This company developed moss-made alpha Galactosidase as a potential treatment for Fabry disease. Moss-aGAL is the first moss-made drug to successfully pass clinical phase 1. Moss-FH is the second candidate pharmaceutical in the pipeline and many more proteins will follow. I find it very satisfying to develop a biological system, to answer fundamental questions in biology and at the same time develop new products for the benefit of humankind.
Contact Info for Prof. Dr. Ralf Reski
Chair Plant Biotechnology
Faculty of Biology
University of Freiburg, Germany
E: ralf.reski@biologie.uni-
Homepage: http://plant-biotech.net/
Know More
The production of biologically active human factor H in moss (moss-FH) was reported in:
Büttner-Mainik, A., J. Parsons, H. Jérome, A. Hartmann, S. Lamer, A. Schaaf, A. Schlosser, P.F. Zipfel, R. Reski, E.L. Decker (2011): Production of biologically active recombinant human factor H in Physcomitrella. Plant Biotechnology Journal 9, 373-383. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1467-7652.2010.00552.x
The successful preclinical trials of moss-FH were subsequently reported in:
Häffner, K., J. Parsons, L.L. Bohlender, S. Hoernstein, H. Niederkrüger, B. Fode, A. Busch, N. Krieghoff, J. Koch, A. Schaaf, T. Frischmuth, P.F. Zipfel, M. Pohl, R. Reski, E.L. Decker, S. Michelfelder (2017): Treatment of experimental C3 Glomerulopathy by human complement factor H produced in glycosylation-optimized Physcomitrella patens. Molecular Immunology 89, 120.
Michelfelder, S., J. Parsons, L.L. Bohlender, S.N.W. Hoernstein, H. Niederkrüger, A. Busch, N. Krieghoff, J. Koch, B. Fode, A. Schaaf, T. Frischmuth, M. Pohl, P.F. Zipfel, R. Reski, E.L. Decker, K. Häffner (2017): Moss-produced, glycosylation-optimized human factor H for therapeutic application in complement disorders. Journal of the American Society of Nephrology 28, 1462-1474. http://jasn.asnjournals.org/content/early/2016/12/07/ASN.2015070745.abstract
Click below to view the presentation about Moss-FH
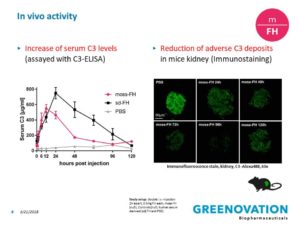
MOSS-MADE-PHARMACEUTICALS-Moss-FH
A novel synthetic fusion protein (MFHR1) was published in:
Michelfelder, S., F. Fischer, A. Wäldin, K.V. Hörle, M. Pohl, J. Parsons, R. Reski, E.L. Decker, P.F. Zipfel, C. Skerka, K. Häffner (2018): The MFHR1 fusion protein is a novel synthetic multitarget complement inhibitor with therapeutic potential. Journal of the American Society of Nephrology 29, DOI: 10.1681/ASN.2017070738. http://jasn.asnjournals.org/content/early/2018/01/14/ASN.2017070738
In 2015, the journal Nature Biotechnology reported on the first moss-made drug, alpha Galactosidase (moss-aGAL) to treat Fabry Disease: https://www.nature.com/articles/nbt1115-1122a
The company that developed and markets the proprietary moss technology is the privately held Greenovation Biopharmaceuticals in Germany: http://www.greenovation.com/
Information about their lead product moss-aGAL can be found here:
http://www.greenovation.com/moss-agal-agalsidase.html
Information about moss-FH can be found here:
http://www.greenovation.com/moss-fh-factor-h.html
Greenovation product pipeline is described here:
http://www.greenovation.com/developmental-pipeline.html
Publicly available brochures and pictures from Greenovation can be obtained here:
http://www.greenovation.com/press-kit.html
A current review article about moss technology and moss-made pharmaceuticals can be found here:
Reski, R., J. Parsons, E.L. Decker (2015): Moss-made pharmaceuticals: from bench to bedside. Plant Biotechnology Journal 13, 1191-1198. https://onlinelibrary.wiley.com/doi/full/10.1111/pbi.12401

