
As COVID-19 research, vaccines & availability, and national/regional response vary greatly by day and location, aHUS Alliance Global Action reminds you to check with your medical team for advice and updates.
Original content, in our 2021 and 2022 articles on aHUS & COVID-19 continued to focus on matters of high interest to they atypical HUS community, to include a March 2020 webinar on this topic. By nature scientific information continually changes, so we encourage you to stay current regarding this and any aHUS topic of interest. See our Research & Publications page for more scientific studies and reviews for more about atypical HUS, and if you wish to view more research specific to COVID-19 please look under the categories for ‘Treatment’ and also ‘Triggers’.

Our Articles – a sampling of 2022 Original Content on aHUS & COVID, from our Website. (see more below)
What about aHUS and COVID Vaccinations and access to genetic testing and genetic research (April 2022)
Questions about aHUS Triggers (April 2022)
COVID Triggers aHUS, literature yield evidence (March 2022)
Long aHUS (Feb 2022)
Updated 1 January 2023 – sampling of COVID-19 research directly related to Atypical HUS
Bouwmeester et al. COVID-19 vaccination and Atypical hemolytic uremic syndrome (Dec 2022)
Ul Abedin et al. COVID-19 Associated Atypical Hemolytic Uremic Syndrome (Nov 2022)
Rysava et al. Atypical hemolytic uremic syndrome triggered by mRNA vaccination against SARS-CoV-2: Case report (Sept 2022)
Smarz-Widelska et al Atypical Hemolytic Uremic Syndrome after SARS-CoV-2 Infection: Report of Two Cases (Sept 2022)
Claes et al Atypical Hemolytic Uremic Syndrome Occurring After Receipt of mRNA-1273 COVID-19 Vaccine Booster: A Case Report (Sept 2022)
Ahamed and Laurence Long COVID endotheliopathy: hypothesized mechanisms and potential therapeutic approaches (Aug 2022)
Tiwari et al Atypical HUS Triggered by COVID-19: A Case Report (May 2022)
Quekelberghe et al Atypical hemolytic uremic syndrome induced by SARS-CoV2 infection in infants with EXOSC3 mutation (May 2022)
Leone et al Case Report: Tackling Complement Hyperactivation With Eculizumab in Atypical Hemolytic Uremic Syndrome Triggered by COVID-19 (Feb 2022)
Hamza Severe SARS-COV-2 infection in pediatric patient with atypical Hemolytic Uremic Syndrome: A case report (Feb 2022)
Aiello et al C5a and C5aR1 are key drivers of microvascular platelet aggregation in clinical entities spanning from aHUS to COVID-19 (Feb 2022)
Khandelwal et al. Anti-factor H antibody associated hemolytic uremic syndrome following SARS-CoV-2 infection (Jan 2022)
We’d like to draw your attention to an August 2022 literature review regarding COVID-19 triggering atypical HUS. This article by Boldig et al notes 9 reported case of aHUS triggered by COVID-19, 7 of which were initial presentations (no previous aHUS episoded) and 2 cases where people previously diagnosed with aHUS relapsed.
COVID-19: A Rare Cause of Hemolytic Uremic Syndrome
For more research into all aspects of atypical HUS, to include diagnosis, treatment, complement activation, thrombotic microangiopathy and more:
Click HERE to visit our Research & Publications page.
From Our Earlier Edition of this Article:
While research updates to include COVID-19 & aHUS have been continuously added to our Research & Publications page, here are just a few.
Compiled Array of 15 Medical Publications: aHUS & COVID-19 (posted March 2022)
El Sissy et al COVID-19 as a Potential Triggers for Complement-mediated aHUS
Gill et al COVID-19–associated atypical hemolytic uremic syndrome and use of Eculizumab therapy
(COVID-19) Dalkıran et al Thrombotic Microangiopathy in a Severe Pediatric Case of COVID-19
Bašić‐Jukić et al Additional eculizumab dose and tacrolimus discontinuation for treatment of COVID‐19 in a kidney transplant recipient with aHUS
April 2022
What about aHUS and COVID Vaccinations and access to genetic testing and genetic research?
March 2022
aHUS and COVID 19 Vaccine Update
January 2022
Follow up on aHUS COVID-19 Jabs
aHUS and COVID-19 Vaccinations
December 2021
COVID and aHUS at the end of 2021
July 2021
March 2021
aHUS and COVID-19 Vaccine Update
January 2021
aHUS and COVID-19 Vaccinations
The aHUS Alliance has created this page of resources as an initial place for the atypical HUS community to learn more about issues surrounding this rare disease during the COVID-19 pandemic. Information about the coronavirus is fast-paced and ever-evolving, and which would make resource lists such as this incomplete, and in need of updated information, from the very moment ours was created (30 March 2020) to highlight topics of high interest in the aHUS global community.
We caution that new information about the coronavirus is learned on an almost hourly basis, so aHUS patients and family caregivers are strongly advised to regularly visit their national government’s outlets for health updates, COVID-19 guidelines, and details related to infectious disease spread in their regions.
Heath care policy, resource allocation, treatment options, drug access, and medical care situations vary widely around the world for those living with aHUS. Given that, and the varied clinical profiles of aHUS patients, it would be almost impossible that any one particular medical protocol would be advised for those aHUS patients battling the coronavirus.
Some nations with aHUS/TMA study centres have released information specifically for this rare disease, and there are medical associations which have offered targeted information of high interest to include kidney transplants or dialysis. COVID-19 information will evolve over time, which should bring new knowledge and necessitate frequent updates as the situation unfolds.
Updates are clearly marked, with our final COVID-19 updates added 16 December 2020. Information and links appear in date order, newest to older posts and added by month as indicated below:
Important Note:
Information & Research about COVID-19 changes every day, making it essential for people to monitor news in their nation. International collaborations understandably maintain strict control over information about clinical trials, research efforts, and intellectual property relating to COVID-19 discoveries.
To keep abreast of advancements, utilize key search terms including:
convalescent plasma (antibodies)
monoclonal antibodies (synthetic)
COVID-19 & stem cells
Vaccines
Updates for December 2020
COVID-19 Vaccine Information
With the advent of COVID-19 vaccines now administered in multiple nations around the world, the aHUS Alliance Global Action team posts our FINAL update on this page (16 Dec 2020).
Here is a small sample of COVID-19 vaccine news releases by various pharma, but it remains to be determined by individual nations as to what vaccines are employed for their nations and what individuals or groups will receive priority status.
We encourage aHUS patients to check their nation’s policies and updates on COVID-19, and to check with your medical team regarding vaccination questions and availability.
15 Dec 2020
CanSinoBIO: Ad5-nCoV SARS-CoV-2 Vaccine Description
14 Dec 2020
CureVac Commences Global Pivotal Phase 2b/3 Trial for COVID-19 Vaccine Candidate, CVnCoV
14 Dec 2020
Gamaleya Research Institute: THE SPUTNIK V VACCINE’S EFFICACY IS CONFIRMED AT 91.4% BASED ON DATA ANALYSIS OF THE FINAL CONTROL POINT OF CLINICAL TRIALS
10 Dec 2020
INOVIO: INOVIO and Advaccine Announce First Dosing of Subject in Phase 2 Clinical Trial for COVID-19 DNA Vaccine Candidate INO-4800 in China
8 Dec 2020
Oxford–AstraZeneca COVID-19 vaccine efficacy
Dec 2020
Sanofi’s Two Vaccine Candidates against COVID-19
For other pharma with COVID-19 Vaccines in development,
see previous months regarding these companies:
Novavax (NVX‑CoV2373, see Nov 2020)
MERK (CD24Fc, see Nov 2020)
Updates for November 2020
Specific to Atypical HUS
11 Nov 2020
Ville et al. Atypical HUS relapse triggered by Covid-19 (KI Reports)
Case study, 1st reported case of aHUS relapse triggered by COVID-19. “Nevertheless, our observation underlines the need for a close monitoring of aHUS patients who discontinued eculizumab in the setting of COVID-19. It also a further indication that complement blockade should not be discontinued in aHUS during infectious episodes, COVID-19 not being an exception.”
Alizadeh et al. Toddler with New Onset Diabetes & aHUS in the setting of COVID-19 (Nov Issue: Pediatrics)
Case study, ‘likely to be a case of aHUS triggered by COVID-19″ and with multiple complexities. “This is a novel case of a 16-month-old male with a history of prematurity with
intrauterine growth restriction, severe failure to thrive, microcephaly, pachygyria, agenesis of the
corpus callosum and postnatal embolic stroke, who presented with new-onset diabetes mellitus
with diabetic ketoacidosis in the setting of SARS-CoV-2 infection, with a course complicated by
atypical hemolytic syndrome (aHUS).”
Covid-19 Info of interest to the aHUS community
30 Nov 2020
Novavax Announces COVID-19 Vaccine Clinical Development Progress
23 Nov 2020
AstraZeneca: AZD1222 vaccine met primary efficacy endpoint in preventing COVID-19
23 Nov 2020
MERK: Merck’s SARS-CoV-2/COVID-19 Research Efforts: Timeline
22 Nov 2020
Vaccine News – AstraZeneca & University of Oxford announced its positive results from their Phase III trials that show their candidate vaccine, ChAdOx1 nCoV-2019, is effective at preventing COVID-19.
Oxford University website: Oxford University breakthrough on global COVID-19 Vaccine
21 Nov 2020
COVID-19 Treatment News – Combination of 2 Drugs approved in the USA to treat mild to moderate COVID-19 in adults, as well as in pediatric patients at 12 years of age. From the Regeneron corporate website,
Regeneron’s Casirivimab And Imdevimab Antibody Cocktail For Covid-19 Is First Combination Therapy To Receive FDA Emergency Use Authorization
16 Nov 2020
Moderna releases 1st interim set of data from their Phase 3 study of the company’s COVID-19 vaccine candidate, mRNA-1273, which indicated a vaccine efficacy of 94.5%. Moderna will submit an ‘Emergency Use Authorization’ to the US FDA in the coming weeks.
Like the 2 dose injection noted by Pfizer/BioNTech COVID-19 vaccine (9 Nov), Moderna’s mRNA-1273 vaccine also utilizes a messenger RNA approach. Moderna’s vaccine is reported to requires less special storage/handling conditions to ease distribution of the vaccine.
FMI: Moderna (corporate site, IR)
BusinessWire, Press Release
15 Nov 2020
Johnson & Johnson Initiates Second Global Phase 3 Clinical Trial of its Janssen COVID-19 Vaccine Candidate
9 Nov 2020
Advancements in mRNA – An Approach to COVID-19 Vaccines (aHUS Alliance, article)
Pfizer & BioNTech announce positive data regarding their COVID-19 vaccine candidate. What’s messenger RNA (mRNA)? The aHUS Alliance compiles info & resources for background on mRNA & its potential to prevent disease.
9 Nov 2020
USFDA issues “emergency use authorization (EUA) for the investigational monoclonal antibody therapy bamlanivimab for the treatment of mild-to-moderate COVID-19 in adult and pediatric patients.”
Read the full press release HERE
Updates for October 2020
Covid-19 Info of interest to the aHUS community
27 Oct 2020
Kant et al The COVID-19 nephrology compendium: AKI, CKD, ESKD and transplantation
Key resource for impact of COVID-19 across a range of nephrology issues – dialysis, transplant, patterns of injury to the kidney, management of immunosuppression, and more. Open access
20 Oct 2020
Francis Collins Two Studies Show COVID-19 Antibodies Persist for Months
USA: Article by the Director of the National Institute of Health (NIH) On the topic of whether those who have experienced COVID-19 infection have acquired immunity against being ill a second time.
12 Oct 2020
Magro et al. Docked SARS CoV-2 Proteins within the Cutaneous and Subcutaneous Microvasculature and their Role in the Pathogenesis of Severe COVID-19
A study to determine if a specific type of skin biopsy can work to assess the status of patients with severe forms of COVID-19. “A deltoid skin biopsy has been used to diagnose patients with other systemic complement-mediated microvascular injury syndromes such as atypical hemolytic uremic syndrome (aHUS) and differentiate these disorders from thrombotic thrombocytopenic purpura associated with antibodies to ADAMSTS13”
Updates for September 2020
Specific to atypical HUS
27 Sept 2020
Trimachi et al Eculizumab, SARS-CoV-2 and atypical hemolytic uremic syndrome
Case Study of an aHUS patient (male age 24) with 6 yr history of kidney transplant: “Microthrombotic biomarkers were absent. One week later he developed respiratory failure, fever and radiological progression of the CT scan infiltrates (Figure 1B), associated with elevated inflammatory biomarkers. In the intensive care unit (ICU), the antibiotic regime was switched to vancomycin and cefepime and hydrocortisone was changed to IV dexamethasone 6 mg/day. A 200-mL convalescent plasma infusion was prescribed. He received oxygen via mask reservoir (5 L/min) and vigil pronation cycles and showed a sustained improvement without the need for mechanical ventilation.”
Other Covid-19 Info of interest to the aHUS community
12 Sept 2020
Clinical Trials for COVID-19
Note: Use the ‘official’ names for research & clinical trials related to the coronavirus: SARS-CoV-2 and 2019-nCoV
COVID-19 Clinical Trials listed on Clinical Trials.gov – Click to view the List of 3296 Studies
3296 Studies as of 12 Sept 2020
15 Sept 2020
Sarkar et al. Potential Therapeutic Options for COVID-19: Current Status, Challenges, and Future Perspectives
A look at potential drugs to treat COVID-19 patients, this review include monoclonal antibodies (such as ravulizumab & eculizumab) as well as other therapeutic drugs. Could aHUS knowledge be expanded by new information about how these drugs work to tamp down complement and reduce inflammation?
Tabbibi et al, Journal of Intensive Care (Sept 2020) Therapeutic Plasma Exchange: A potential Management Strategy for Critically Ill COVID-19 Patients
“The morbidity and mortality of the infection varies based upon patient age, comorbid conditions, viral load, and the availability of effective treatments.” AND
“Therapeutic plasma exchange (TPE) merits consideration in the treatment of critically ill COVID-19 patients and is an avenue for clinical trials to pursue. If efficacious, faster recovery of patients may lead to shorter intensive care unit stays and less time on mechanical ventilation. Herein, we briefly discuss some of the various approaches currently being investigated for the treatment of SARS-CoV-2 with a focus on potential benefits of TPE for selected critically ill patients.”
Updates for August 2020
Specific to atypical HUS
13 Aug 2020
ARTICLE: Like an Agatha Christie Story!
Other Covid-19 Info of interest to the aHUS community
23 Aug 2020
Pediatric Nephrology (journal): Be aware of acute kidney injury in critically ill children with COVID-19 “An inflammatory storm and complement-mediated injury may underlie AKI development in children with COVID-19.”
11 Aug 2020
Nature: Antibody therapies could be a bridge to a coronavirus vaccine — but will the world benefit? “As the race to develop a vaccine against COVID-19 rages on, some researchers are focused on a short-term way to treat people with the disease: monoclonal antibodies.” AND “But mass-produced antibodies, routinely used to treat diseases such as cancer, are complex to manufacture and come with a hefty price tag. That risks placing them beyond the reach of poor countries.”
6 Aug 2020
Java et al (JCI Insight) The complement system in COVID-19: friend and foe?
“We posit that (a) coronaviruses activate multiple complement pathways; (b) severe COVID-19 clinical features often resemble complementopathies; (c) the combined effects of complement activation, dysregulated neutrophilia, endothelial injury, and hypercoagulability appear to be intertwined to drive the severe features of COVID-19; (d) a subset of patients with COVID-19 may have a genetic predisposition associated with complement dysregulation; and (e) these observations create a basis for clinical trials of complement inhibitors in life-threatening illness.”
6 Aug 2020
Clinical Immunology (research): Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19
“Respiratory failure and acute kidney injury (AKI) are associated with high mortality in SARS-CoV-2-associated Coronavirus disease 2019 (COVID-19). These manifestations are linked to a hypercoaguable, pro-inflammatory state with persistent, systemic complement activation.” AND “In conclusion, anti-complement therapy may be beneficial in at least some patients with critical COVID-19.”
4 Aug 2020
Science (magazine): Designer antibodies could battle COVID-19 before vaccines arrive
“While the world is transfixed by the high-stakes race to develop a COVID-19 vaccine, an equally crucial competition is heating up to produce targeted antibodies that could provide an instant immunity boost against the virus. Clinical trials of these monoclonal antibodies, which could both prevent and treat the disease, are already underway and could produce signs of efficacy in the next few months, perhaps ahead of vaccine trials.”
Updates for JULY 2020
Specific to atypical HUS
13 July
ARTICLE: aHUS Health, Well-Being and Work
Data from the aHUS global patient registry includes topics like fatigue and quality of life issues for aHUS patients who have never used eculizumab, as well as during use and post-use of that thereapeutic drug. “Increasingly there are reports that there will be a surge of stress in those survivors of COVID 19 with the trauma of experiencing the severe near death form of the illness.”
Other Covid-19 Info of interest to the aHUS community
1 July 2020
CJASN: COVID-19 Outbreak and Management Approach for Families with Children on Long-Term Kidney Replacement Therapy
Regarding COVID-19 and children on long term dialysis, “The coronavirus disease 2019 outbreak has had physical, mental, logistical, and financial effects on families with children on long-term KRT.”
Updates for JUNE 2020
Specific to atypical HUS
24 June 2020
ARTICLE: aHUS 2020 Therapeutic Drug Landscape (the impact of COVID-19 on aHUS research)
18 June 2020
ARTICLE: German aHUS Patient Day Webinar (in German, held 6 June 2020)
14 June 2020
ARTICLE: aHUS in Deutschland – (With COVID-19 content (in German) from Sebsthilfegruppe aHUS und MPGN
1 June 2020
ARTICLE: The Sun Rises, but It’ll never be the Same Again
Other Covid-19 Info of interest to the aHUS community
18 June 2020
JCI Insights (Journal of Clinical Investigation) The complement system in COVID-19: friend and foe?
Java et al propose that the coronavirus activates multiple complement pathways, perhaps with a COVID-19 patient subset genetically predisposed to complement-mediated diseases.
7 June 2020
Kidney International Thrombotic microangiopathy in a patient with COVID-19
Case history of a 69 year old patient with coronavirus disease (COVID-19), whose medical history included asthma, and diagnosed with thrombotic microangiopathy via a kidney biopsy.
2 June 2020
Campbell and Kahwash. Will Complement Inhibition Be the New Target in Treating COVID-19–Related Systemic Thrombosis?
Updates for MAY 2020
Specific to atypical HUS
8 May 2020
ARTICLE: Reflections on aHUS During a Global Pandemic
6 May 2020
ARTICLE: Thrombotic Microangiopathy & Precision Medicine (What’s to be learned from the Pandemic?)
Other Covid-19 Info of interest to the aHUS community
27 May 2020
BAPN: Updated shielding guidance for children with kidney disease, on dialysis, and immunosuppression (including kidney transplants)
New Guidelines for COVID-19 regarding children in the UK, from the British Association for Paediatric Nephrology. (aHUS can drastically affect kidney function, causing patients to need dialysis or transplant.)
27 May 2020
Journal of Clinical Investigation: COVID-19, microangiopathy, hemostatic activation, and complement
On COVID-19 and complement: “Our understanding of inappropriate complement activation in human disease derives mainly from genetic mutations of complement proteins — either loss-of-function mutations in regulatory proteins that protect host tissues under homeostatic conditions or gain-of-function mutations resulting in resistance to regulatory protein surveillance” AND “The thrombotic microangiopathy (TMA) of the atypical hemolytic uremia syndrome (aHUS) leads to thrombocytopenia, hemolytic anemia, and renal failure. However, thrombotic events in aHUS are not confined to the kidney; 3% to 10% of patients have cardiac complications due to coronary microangiopathy, and patients often develop complications involving other organs. The most frequent mutations in aHUS are found in the regulatory protein factor H (FH), and such mutations lead to MAC-mediated endothelial injury and capillary thrombosis, which are the hallmarks of TMA.”
Multi-System Inflammatory Disease (MISC-C)
20 May 2020
News Media USA, Article on MIS-C (NY Times, not a Research/Medical Source) Young adults are also affected by Kawasaki-like disease linked to coronavirus, doctors say
14 May 2020
CDC (USA Center for Disease Control): For Parents: Multisystem Inflammatory Syndrome in Children (MIS-C) associated with COVID-19
7 May 2020
Kidney International: Naiker et al. The Novel Coronavirus 2019 epidemic and kidneys
Kidney experts weigh in on key issues on how COVID-19 affects the kidneys and management of various clinical presentations of the disease.
5 May 2020
British Journal of Hematology. Severe COVID‐19 infection and thrombotic microangiopathy: success does not come easily
Gavriilaki and Brodsky make the case for why COVID-19 cases should be considered ‘through the prism of TMA’.
Updates for MAY 2020
23 April
Risitano et al. Complement as a target in COVID-19?
“Most patients who become critically ill following infection with SARS-CoV-2, the causative agent of COVID-19, develop acute respiratory distress syndrome (ARDS)1. The deterioration of lung function has been attributed to a maladaptive immune response rather than increased viral loads.” AND
“Complement is a key player of protective immunity against pathogens, but its excessive or deregulated activation may result in collateral tissue injury. However, complement inhibitors are currently only used in rare human diseases, such as paroxysmal nocturnal haemoglobinuria. In these unprecedented times, we would encourage all complement-dedicated pharmaceutical companies, as well as individual scientists, to actively contribute to our efforts to understand the role of complement in COVID-19.”
Updated: 27 April 2020
COVID-19 and Dialysis

Article COVID-19: Challenges faced by Dialysis Patients
A Glimpse into Coronavirus History
SARS-CoV-2: A Perspective
The 1918 influenza pandemic (sometimes called the ‘Spanish Flu’) established the first widespread use of plasma from recovering patients, known as ‘convalescent plasma’ or CBP, which was also adopted for the treatment during the Ebola outbreak in 2013 (see JAMA article). In 2003 the SARS (Severe Acute Respiratory Syndrome or SARS-CoV) outbreak affected an estimated 26 nations, and launched study into coronaviruses as well as into possible vaccines (source: WHO).
COVID-19 like SARS or MERS is a disease caused by a form of coronavirus, a previously unknown virus known as SARS-CoV-2 which causes the disease COVID-19 (“Co” refers to corona, “vi” to virus, “d” to disease, and “19” for 2019). Reports from the frontlines of the COVID-19 outbreak note utilization of convalescent plasma, or an infusion of plasma from people previously recovered and containing antibodies against this coronavirus, as a potential therapeutic for patients seriously ill with COVID-19. As information and research unfold, and evidence-backed data rolls in, new therapeutic options and socio-economic impact will continue to have global focus now and for future generations.
Updated: 17 April 2020
COVID-19 Info & Resources

17 April 2020 – It’s a difficult task to keep track of COVID-19 news & research, especially since it’s a continually evolving situation. Here’s a selection of info (research & articles) that may be of particular interest to people interested in atypical HUS. Scroll to the end of these aHUS Alliance COVID-19 updates for resources specifically related to atypical HUS.
Mental Heath: Issues & Resources
Mental Health & COVID-19 – aHUS Alliance Resource Page (Article)

COVID-19 & Complement
Note: The complement system is a part of the body’s immune system that enhances (complements) our ability to prevent or defend against infections.
Coronaviruses hijack the complement system (Article by Wilk, CM, 14 Apr 2020)
Background for our Readers: CM-TMA article
Complement-mediated TMA Awareness – Did you know it was aHUS?
Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases (Magro et al, 15 Apr 2020)
Specific Drugs & Treatments in the News
During the 24 March 2020 webinar for FAQs about atypical HUS & COVID-19, Dr. Marie Scully (UK) noted that there were an estimated 300 drugs of interest as potential therapeutics under study. New information appears almost hourly, so your review of updated information is critical.
Overviews
Here’s What We Know about the Most Touted Drugs Tested for COVID-19 (16 Apr 2020, Scientific American)
Drug Evaluation during the Covid-19 Pandemic (14 Apr 2020, NEJM)
Clinical Trials for COVID-19
Note: Use the ‘official’ names for research & clinical trials related to the coronavirus: SARS-CoV-2 and 2019-nCoV
COVID-19 Clinical Trials listed on Clinical Trials.gov – List of 657 Studies
657 Studies as of 17 April 2020
Mentioned in the Media
COVID-19 Convalescent Plasma Therapy (use of plasma antibodies from people already recovered from coronavirus) Convalescent Plasma: What is it?
Convalescent plasma: Possible treatment for COVID-19? (10 Apr 2020, Mayo Clinic)
COVID-19 and Convalescent Plasma: Frequently Asked Questions (10 Apr 2020, ASH)
Remdesivir (antiviral, originally developed by Gilead Sciences to treat Ebola & Marburg virus diseases)
Gilead & COVID-19 Clinical Trials
NEW Info & Data from Gilead Sciences CEO: 29 April 2020
Hydroxychloroquine (not to be confused with chloroquine) was originally developed to prevent & treat malaria, but is currently used for rheumatoid arthritis, lupus, and other conditions.
Now studied for COVID-19, and considered in a combination of hydroxychloroquine & azithromycin.
NIH Study (USA)
Icatibant
NL: Researchers at the Radboud UMC look at Bradykinin as a mechanism for COVID-19 blood leakage to impact lung function, and explore use of Icatibant to inhibit bradykinin, in the EU for HAE.
Article regarding impact of this research
Research – Kinins and Cytokines in COVID-19: A Comprehensive Pathophysiological Approach (3 Apr 2020)
Many others under consideration so check: COVID-19 Clinical Trials listed on Clinical Trials.gov 657 Studies as of 17 April 2020
Vaccines Against COVID-19
Of the many companies and academic institutions with a COVID-19 vaccine in development, 5 are currently testing their vaccine candidates in humans. Moderna was the first of these to begin human trials on 16 March.
On 17 April 2020, 47 studies for COVID-19 vaccines were found on ClinicalTrials.gov using these search terms: SARS-CoV-2, Vaccination, and Immunization
Public statement for collaboration on COVID-19 vaccine development (13 Apr 2020, WHO)
Coronavirus vaccine: when will we have one? (15 Apr 2020, The Guardian)
Moderna Ships mRNA Vaccine Against Novel Coronavirus (mRNA-1273) for Phase 1 Study (24 Feb 2020, via Moderna)
INOVIO Initiates Phase 1 Clinical Trial Of Its COVID-19 Vaccine and Plans First Dose Today (6 Apr 2020, via INOVIO)
Novavax Identifies Coronavirus Vaccine Candidate; Accelerates Initiation of First-in-Human Trial to Mid-May (via Novavax)
Sanofi & GSK to join forces in unprecedented vaccine collaboration to fight COVID-19 ( 14 Apr 2020, via GSK)
Atypical HUS & COVID-19
Scroll Down for Details & Original Posts
UK – National aHUS Service (Home Page: “Important info about COVID-19”)
NL – CUREiHUS (Guidelines: Original in Dutch or Translate into preferred language.)
aHUS & COVID-19 – Webinar (Video & Transcript: FAQ w/ patients, the Global aHUS Registry)
aHUS Alliance: Articles on COVID-19
8 March 2020 aHUS Trials Watch 10 Toronto Complement Conference, NCT04288713, and COVID-19
14 Mar 2020 They “Get It’ When they can Get It Parallels among COVID-19 & Rare Diseases
18 Mar 2020 aHUS and COVID-19 Conversations and Insights
Not Familiar with the rare disease Atypical HUS?

Continue to SCROLL DOWN to View
Resource Posted Prior to Our Updates (Above)
Note: The aHUS Alliance does not provide medical advice nor promote or suggest medical treatment or care. Information and resources here portray a small selection of what is available to medical professionals, and is no substitute for the advice given to patients by their own physicians. Should you suspect exposure to COVID-19, contact your doctor and local healthcare services for updated directives.
Start Learning: Atypical HUS & COVID-19
aHUS Alliance – Articles
8 March 2020 aHUS Trials Watch 10 Toronto Complement Conference, NCT04288713, and COVID-19
14 Mar 2020 They “Get It’ When they can Get It Parallels among COVID-19 & Rare Diseases
18 Mar 2020 aHUS and COVID-19 Conversations and Insights
Not Familiar with the rare disease Atypical HUS?
24 March 2020 Webinar: Atypical HUS & COVIDー19
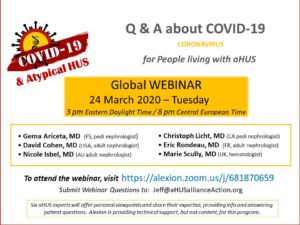
A panel of 6 aHUS experts from the Scientific Advisory Board (SAB) of the global aHUS registry shared info and answered questions from patients and aHUS family caregivers regarding the effects of coronavirus on those diagnosed with this very rare disease.
We are extremely grateful for the physicians who shared their time and expertise as webinar presenters during their pressing professional duties during the COVID-19 outbreak: Gema Ariceta, MD, Spain (pediatric nephrologist), David Cohen, MD, USA (adult nephrologist), Eric Rondeau, MD, France (adult nephrologist), Marie Scully, MD, United Kingdom (hematologist), and panel moderator Christoph Licht, MD, Canada (pediatric nephrologist).
Over 300 people attended the webinar in real time, and now we are pleased to announce that archived video from this 24 March 2020 ‘aHUS & COVID-19’ webinar has become available. Our thanks to Alexion for providing the IT support for the webinar.
Click to read the ARTICLE describing the Webinar
Click the Webinar Image Below to WATCH the Video

Click to download Transcript (pdf) aHUS & COVID19, Webinar Transcript 24 Mar 2020 Courtesy of the Global HUS Registry SAB (permission granted by Dr C Licht)
Guidelines from aHUS centres of Expertise & Research
UK National aHUS Service (In affiliation with the NHS, National Renal Complement Therapeutics Centre & Newcastle Hospitals).
Posted on their website’s Home Page, which indicated regular information updates
http://www.atypicalhus.co.uk/
NL CUREiHUS (In affiliation with the Dutch Radboudumc Expertise Center Rare Kidney Diseases, CUREiHUS is a national study for to determine optimal treatment for aHUS)
http://cureihus.nl/nieuws/covid-19-coronavirus-en-ahus/
Atypical HUS in Critical Care Settings: Medical Publications
Azoulay et al (2017) Expert Statements on the Standard of Care in Critically Ill Adult Patients With Atypical Hemolytic Uremic Syndrome
Manrique-Caballero et al (2020) Typical and Atypical Hemolytic Uremic Syndrome in the Critically Ill
Rivera et al (2018) Impact of a multidisciplinary team for the management of thrombotic microangiopathy
Vincent et al (2018) Thrombocytopenia in the ICU: disseminated intravascular coagulation and thrombotic microangiopathies—what intensivists need to know
See more topic specific research at the
aHUS Alliance list of aHUS Publications
New publications on the general public & COVID-19 are continually being released. One example regarding Critical Care is below:
Poston et al (26 Mar 2020) Management of Critically Ill Adults With COVID-19
Many associations have offered information during the 2020 COVID-19 outbreak, to include medical professionals and specialists in fields of interest to the aHUS community.
Here are some examples of resources available, but many more exist to aid physicians and the public. Many are nation-specific, so we caution readers to look for dates on materials to ensure that the COVID-19 information is updated – and to expand online research to find resources both in your nation and within international organizations.

Updated 6 April 2020
COVID-19 Info & Resources
Coronavirus: World Updates
ECDC (EU Centre for Disease Prevention and Control): World Epidemiological update
Situation update Worldwide, as of 6 April 2020
WHO (World Health Organization): (5 April 2020)
Coronavirus disease 2019 (COVID-19) Situation Report #76
Overviews of COVID-19 Testing & Biotech Efforts
M Palmer, Small companies step up to big role in Covid-19 (20 March 2020) Various companies & COVID-19 tests. Article graphic notes days from exposure to illness and recovery, while the article discusses COVID-19 testing in light of SARS antibody testing for IgG & IgM.

BIO.org Current situations & initiatives in industry (by Company Name) in the fields of: Diagnostics, Therapeutics, Vaccines Biopharmaceutical Innovators Lead the Charge in Fight Against Coronavirus
Stay Home, Stay Safe, Stop the Spread – Media
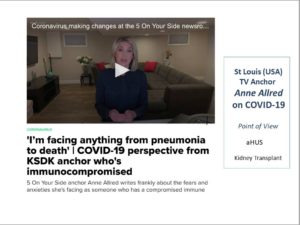
TV Journalist with aHUS, on COVID-19 (Video & Article) KSDK ‘5 On Your Side’ anchor Anne Allred writes frankly about the fears and anxieties she’s facing as someone who has a compromised immune system
Fabric Face Coverings, No Sew Some nations have recommended use of a fabric face covering during the COVID-19 pandemic, and many resources exist online with Do-It-Yourself directions. (Important Note Fabric face coverings cannot match effectiveness or replace medical grade face masks;)
One example of how to make a ‘No Sew’ washable, reusable fabric face covering, using a scarf/handkerchief and two elastic bands, is demonstrated here by Japanese Creations. Video https://youtu.be/EAj12GKuAEk Article & Written directions (EN): http://blog.japanesecreations.com/no-sew-face-mask-with-handkerchief-and-hair-tie
Physical Distancing – Not convinced that it’s important to stop the spread of COVID-19? Based in Los Angles, artist Juan Delcan with partner V. Izaguirre made a 1:40 video of the ‘Matchstick People’ going about their lives, until at 1:20 mins. In a row of burning matches, one Matchstick steps away to stop the fire’s spread. https://vimeo.com/402423439
OTHER Languages: COVID-19 Info
COVID 19: En Français
French Healthcare Network for Rare Immune Hematological Diseases (MaRIH)
Maladies Rares Immuno-Hématologiques Et Coronavirus **Mise À Jour**
Edité par le centre de référence des Microangiopathies Thrombotiques (SHU Atypique)
RECOMMANDATIONS VIS-A-VIS DU COVID19 SPECIFIQUES AUX MAT
FR COVID-19 & Transplants (4 Apr 2020, Agence de la Biomédecine Update) Point de situation au 14 avril 2020 Activité de prélèvement et de greffe d’organes et de tissus durant l’épidémie de COVID 19
FR Haut Comité de la santé publique COVID-19 (en français) Resource Hub: High Council of Public Health
COVID 19: En Español
CDC: Lo que necesita saber sobre la enfermedad del coronavirus 2019 (COVID-19)
CDC: Preguntas frecuentes, Enfermedad del coronavirus 2019 (COVID-19)
COVID 19 on Vimeo – Multiple Languages (via USA, Portland ORE)
አማርኛ (Amharic) https://vimeo.com/402796177
العربية (Arabic) https://vimeo.com/402792787
Español (Spanish) https://vimeo.com/402794380
中文 (Chinese, Mandarin) https://vimeo.com/402794967
繁體中文 (Chinese, Cantonese) https://vimeo.com/402794967
فارسی (Farsi) https://vimeo.com/402796902
Français (French) https://vimeo.com/402799212
日本語 (Japanese) https://vimeo.com/402797380
한국어 (Korean) https://vimeo.com/402798518
русский (Russian) https://vimeo.com/402796177
ภาษาไทย (Thai) https://vimeo.com/402793772
Tiếng Việt (Vietnamese) https://vimeo.com/402792292
Previously Posted
COVID-19 Info & Resources
Coronavirus: COVID-19 General Information
WHO (World Health Organization): Coronavirus disease (COVID-19) Pandemic
NEPH JC A ‘living resource’ for nephrologists during the COVID-19 pandemic
ASH (American Society of Hematology) COVID-19 Resources
WHO (World Health Organization): Coronavirus disease (COVID-19) Pandemic
European Centre for Disease Prevention and Control: an Agency of the EU COVID-19
JAMA Network: Coronavirus Disease 2019 (COVID-19) Resource Center
WHO – Coronovirus Symptoms
NHS – COVID-19: Dos & Don’ts
CDC– Schools, Workplaces & Community Locations
CDC – Coronavirus: Getting Ready at Home
National Kidney Foundation: Kidney Patient Prep for Coronavirus
KidneyCare UK: Coronavirus (COVID-19) Guidance for patients with Kidney Disease
Renal & Urology News: Dialysis Facilities Brace for COVID-19
CDC: Pregnancy & Breastfeeding, COVID-19 FAQ
RCOG: Pregnancy & COVID-19
Image from
ISN: Kidney International Naicker et al (2020) The Novel Coronavirus 2019 epidemic and kidneys
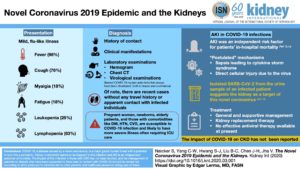
Pediatrics
26 Mar 2020, pdf – UK, from BAPN (British Assn, Pedi Neph) & the Renal Ass’n.
Information and Guidance for Children on Haemodialysis, Peritoneal Dialysis and Immune suppression (including Renal Transplants)
COVID-19 in Kids: Don’t Forget the Bubbles. An Evidence Summary of Paediatric COVID-19 Literature
Transplants
Global Transplantation Covid Report March 2020 The Transplantation Society, journal
Canadian Society of Transplantation: COVID-19 Information
British Transplantation Society: COVID-19 Information
USA, United Network for Organ Sharing (UNOS): COVID-19 Information
American Society of Transplantation: FAQ for Transplants & Coronavirus
Important Notes:
Review dates when COVID-19 information has been posted, to assure that you’re receiving the most updated and accurate details.
The aHUS Alliance suggests that you utilize online translation sites or apps to ensure that you’re viewing updated COVID-19 information, rather than depending on material that may have been translated days ago – and has become out-of-date.
Contact your medical team if you are experience what may be symptoms of COVID-19. The aHUS Alliance, a global group of patient organizations in over 30 nations, cannot provide medical advice.
Physicians treating aHUS patients can reach out to colleagues for online professional support with the aHUS Alliance network of clinicians and investigators. Patients and aHUS family caregivers are encouraged to reach out to their national aHUS organization or to learn about the aHUS Alliance R.O.W. program to support new patient groups.
This resource page was created on 30 March 2020.

Info@aHUSallianceAction.org
