Intracellular complement, or “complosome,” refers to the complement system’s activity within cells, a discovery that has significantly expanded the understanding of complement biology. Traditionally, the complement system has been viewed as a series of proteins circulating in the bloodstream, operating exclusively in extracellular environments to target pathogens. However, recent studies have shown that complement components can also function inside cells, where they play critical roles in processes like metabolism, immune signaling, and cell survival. This intracellular complement activity has profound implications, particularly for organs like the kidney, which are vulnerable to inflammation and immune dysregulation.
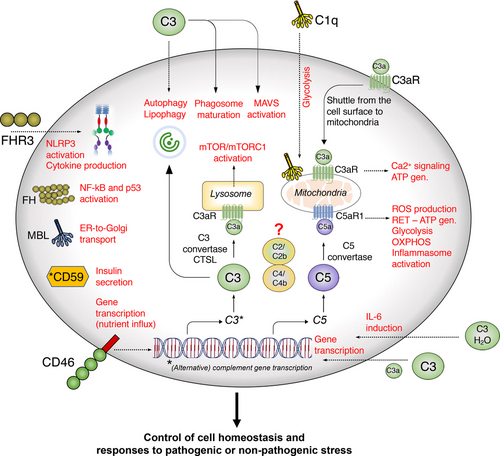
Overview of Intracellular Complement (Complosome)
Intracellular complement involves the presence and activation of complement proteins within cells, independent of the extracellular complement cascade. One of the central proteins in this system is C3, a key component of the complement system that can be cleaved into active fragments (C3a and C3b) within cells. These fragments can bind to cellular receptors or activate intracellular pathways that influence immune responses, cell survival, and autophagy (the process by which cells remove damaged components).
C3 is not the only complement protein involved in intracellular complement. Other complement components, such as complement receptors (e.g., CR3 and C5aR1), have also been found to be expressed within cells. Once activated, these intracellular complement components can regulate various cellular functions, such as the release of cytokines, modulation of metabolic processes, and interaction with cellular machinery involved in autophagy and apoptosis (programmed cell death).
Intracellular Complement and Kidney Function
The kidney is an organ with complex filtration mechanisms that render it particularly vulnerable to immune-mediated damage. The glomeruli, small filtering units within the kidney, are composed of several specialized cell types, including endothelial cells, podocytes, and mesangial cells, which all play a crucial role in maintaining the kidney’s filtration barrier. When intracellular complement pathways become dysregulated, these cells can be significantly affected, leading to a range of kidney pathologies.
1. Podocyte Damage and Proteinuria
Podocytes are specialized epithelial cells that line the glomerular basement membrane and play a critical role in maintaining the integrity of the filtration barrier. Evidence suggests that intracellular complement activity, particularly through C3, can disrupt podocyte function. When intracellular C3 activation is dysregulated, podocytes may undergo apoptosis or abnormal autophagy, leading to structural damage to the filtration barrier. This can result in proteinuria, where proteins such as albumin leak into the urine, a hallmark of kidney diseases like glomerulonephritis and nephrotic syndrome.
The complement system’s intracellular components can also influence the actin cytoskeleton within podocytes, leading to altered cell morphology and detachment from the glomerular basement membrane. This process contributes to the development of focal segmental glomerulosclerosis (FSGS), a disease characterized by scarring of the kidney’s filtration units.
2. Endothelial Cell Dysfunction and Inflammation
Endothelial cells line the blood vessels within the glomeruli and are key regulators of vascular homeostasis. Dysregulated intracellular complement activity in these cells can contribute to endothelial dysfunction, a condition marked by increased vascular permeability, thrombosis, and inflammation. In kidney diseases such as atypical hemolytic uremic syndrome (aHUS), intracellular complement dysregulation leads to endothelial injury, microvascular thrombosis, and subsequent kidney damage.
Intracellular complement, particularly through the activation of the C5a receptor (C5aR1), can also drive inflammatory responses within the kidney. The C5aR1 signaling pathway can amplify the release of pro-inflammatory cytokines and chemokines, leading to local inflammation, recruitment of immune cells, and subsequent tissue damage in the glomeruli. This excessive inflammatory response is particularly detrimental to the kidney, which is highly sensitive to immune-mediated damage.
3. Autophagy and Cellular Stress Responses
Autophagy is a crucial process for maintaining cellular health by removing damaged organelles and proteins. Intracellular complement activation, especially through C3, has been shown to influence autophagy pathways. In the kidney, dysregulation of autophagy can contribute to the accumulation of cellular debris and damaged proteins, leading to cell stress and eventual cell death. Impaired autophagy has been implicated in various kidney diseases, including diabetic nephropathy and ischemia-reperfusion injury, where cells are exposed to metabolic stress and hypoxia.
The relationship between intracellular complement and autophagy is bidirectional. While complement proteins can regulate autophagy, dysregulated autophagy can also influence intracellular complement activation, creating a vicious cycle of cellular damage that is particularly harmful in organs like the kidney. This interplay can lead to chronic inflammation and fibrosis, driving the progression of kidney diseases to end-stage renal failure.
Conclusion
The discovery of intracellular complement activity has opened new avenues for understanding immune regulation within cells. In the context of kidney function, intracellular complement dysregulation can lead to a cascade of harmful effects, including podocyte damage, endothelial dysfunction, and impaired autophagy. Given the kidney’s vulnerability to immune and inflammatory processes, these disruptions are particularly damaging, contributing to the development and progression of various kidney diseases such as glomerulonephritis, aHUS, and diabetic nephropathy.
Targeting intracellular complement pathways offers a promising therapeutic approach for treating complement-mediated kidney diseases. By regulating intracellular complement activation, it may be possible to mitigate the inflammatory and degenerative processes that underlie many forms of kidney damage, potentially improving outcomes for patients with chronic kidney disease.
More information and details available from West and Kemper HERE. Also Singh and Kemper HERE
Illustration from West and Kemper
““The most exciting phrase to hear in science, the one that heralds the most discoveries, is not ‘Eureka’ but ‘That’s funny’….” – Isaac Asimov”
Article No. 692

