The medical community may not agree on everything but all agree on modifying the disease name aHUS..
A report published in 2024 confirmed that.
Now it has to be implemented and each and every current aHUS patient needs to be told what they now have if no longer it is aHUS.
All new patients will need to be told what they have too but what ever they are told it will not be that they have aHUS as past patients were.
These will be one to one conversations between doctor and patient.
This will go smoother if the doctor is fully informed and the patient aware.
As many as 20, 000 or more such conversations involving current “aHUS” patients as well as around 4000 conversations with new patients each year going forward.
But there is much more talking to be done before that as implementation is to be planned for and executed.
Consider this implementation model for modifying aHUS which was included in the published report that said aHUS must go ( i.e. modifying sufficiently so that it is no longer is used except historically!).
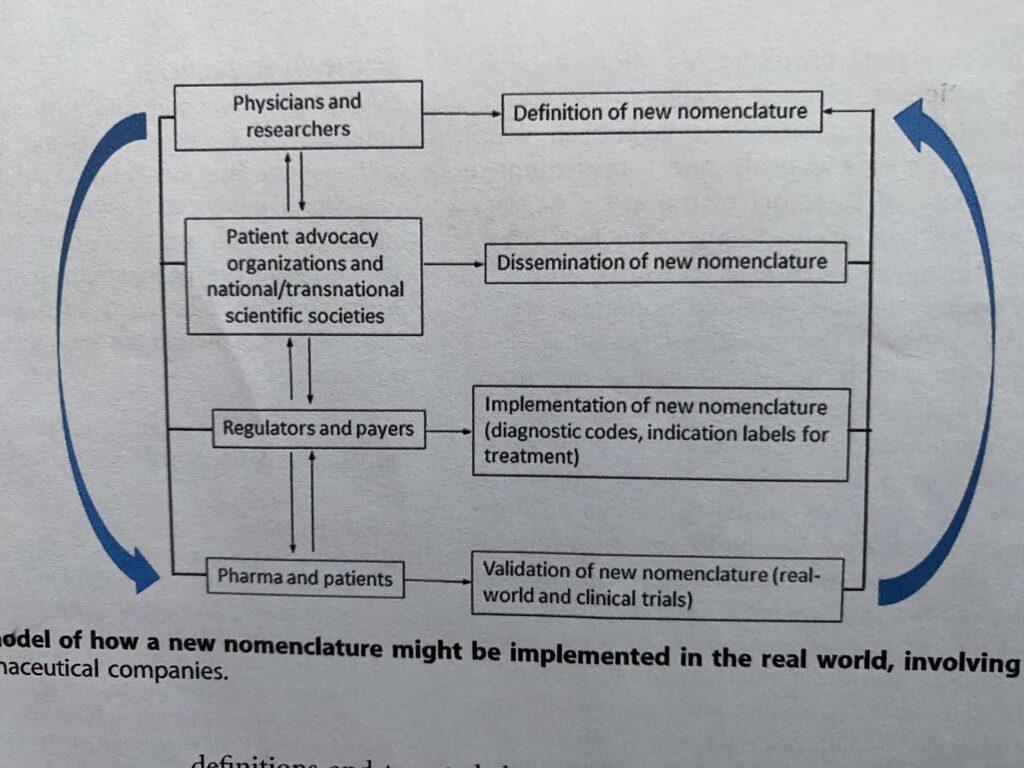
But where do patients fit into all this .
Up to now hardly at all.
The published report acknowledges that as well as Global Action’s thoughtful feedback on the article before it was published and states that more needs to follow.
Patients are definitely stakeholders in all this. They can be beneficiaries, or not, depending on what happens next.
So is it simply a matter of communication channels between “stakeholders”?
The decision makers, Physicians/Researchers, are at the top with immediate two way communication with various global platforms run by patients and professional associations and other medical networks. Both national and global.
These platforms will be used to disseminate the new names decided on by the Physicians/Researchers.
Another indirect communication link is from Physicians/ Researchers to Pharma and Patients.
This is where the new names are put into day to day medical practice.
As part of that day to day practice both Pharma and Patients will validate whether the application of the new categories is correct. Meaning that after being recategorized patients will have got the right diagnosis , continued access to the right treatment and have the right prognosis for future medical management.
And on the right clinical trial as inclusion and exclusion criteria are redefined.
Pharma and Patents will also have an important two way dialogue with Regulators and Payers.
Regulators e.g. FDA and payers private and public will also have a two way dialogue with patient and professional organisations. All sorts of administrative changes will be needed e.g. changing the wording of inhibitor vial leaflet inserts.
And all stakeholders’ issues will in some way or another loop back any issues to the TMA categorisation decision makers.
And the loop begins again in a continuous iterative process going forward as either corrections are needed or new science findings modify what has been agreed previously.
That is the theory. The practice that is another matter.
How practically can the patient voice be heard? Something to think about!
Article No. 707
Previous article on topic:
Change of name for aHUS news
What does the internet have to say about news of change of name for aHUS? Global Action asked it, has the name aHUS been changed. This is the answer it…

