Article No. 436
21 May 2021
It is amazing sometimes what you can find on the internet when searching for something else about aHUS. Recently some research was found in which an attempt was made to find a way for patients to give feedback on what it is like to use the treatments for their illness. This research was done in The Netherlands and is based on two orphan drug treatments, one of which was eculizumab for aHUS.
There are two articles about the way feedback questionnaires were developed and then validated in a live trial, these can be read HERE and HERE.
The Merel Kimman Group set out to develop specific questionnaires about patient expectations and experience of treatments for Idiopathic Pulmonary Fibrosis and aHUS, as well as a generic questionnaire for any diseases. The research group included Marjorie Storm from the Dutch aHUS Patient Knowledge Group.
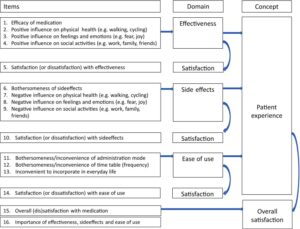
Having established in published literature what satisfaction measures existed , the researchers talked to patients in groups ( 8 aHUS patients were involved ) and in individual interviews. The emerging themes were captured – perceived effectiveness , side effects , ease of use and impact on patients lives.- and two disease specific questionnaires and one generic questionnaires were developed. It became known as the Patients Experience Satisfaction with Medicines or PESaM
The Group then tested the validity of the questionnaires on 188 patients including 11 aHUS patients. They used the established measure EQ- 5D and undertook complex statistical analysis to establish whether the scores were statistically valid. They found the PASaM measure was valid but it needed further application ,and it did.
It was subsequently used by the IPF patient organisation to assess a satisfaction score for IPF ( report HERE) on two of the treatments for IPF, but it is not known if it was similarly performed for aHUS patients.
Below is an example of disease specific PESaM for aHUS, and the issues which would be scored by participating patients. How would the reader rate them 0 to 4?
Below the table are some of the comments made by Dutch aHUS patients about eculizumab and aHUS and included in the published article.
Aside from the aHUS facet, these two manuscripts really highlight the complexity of, and the thinking which goes into , designing a research instrument like a health questionnaire if it is to be used to provide evidence to health decision making authorities or even Pharma.
PESaM
| Category | Responses |
|---|---|
| Effectiveness | Recovery of the body |
| Stable health | |
| Staying alive | |
| Prevent recurrence of disease | |
| More energy | |
| Side effects | Hair loss |
| Bruising | |
| Vomiting | |
| Nausea | |
| Tremor | |
| Muscle pain | |
| Pain in the legs | |
| Fatigue | |
| Blurry vision/vision impairment | |
| Lack of energy | |
| Moody | |
| Risk of meningitis | |
| Risk of infections | |
| Ease of use | Frequency of hospital visits |
| Intravenous insertion | |
| Administration not possible at home | |
| Impact on everyday life | Physical |
| Better fitness levels | |
| Feeling better/less sick | |
| Return to ‘old’ life before illness | |
| Emotional | |
| Life changing/avoiding death | |
| Unknown long-term harms of treatment (worry) | |
| Feeling protected against the disease (reassurance) | |
| Social | |
| Participate in family life | |
| Participation in society | |
| Avoiding busy public spaces | |
| Active social life | |
| Preventing dialysis or kidney transplant | |
| Other | |
| Need for immediate access to antibiotics | |
| Avoiding treatments with increased risk of infection | |
| Change diet (avoid certain foods) |
Comments by patients.
“I don’t have enough energy to work full-time, but I can care for my young son again and do some voluntary work” (female, 37 years).
Side effects that respondents experienced were nausea, vomiting, bruises, hair loss, joint pain, tremor, pain in the legs, fatigue, a moody feeling and loss of sight. Fatigue was most debilitating in the first days after receiving the therapy. Respondents did find it difficult to separate side effects from actual symptoms of aHUS. Furthermore, patients worried about possible long-term sequelae and their increased risk of infection. Because of their fear of infections, some respondents avoided busy public places and food they felt would impose a higher risk of infections.
“I avoid airplanes and air-conditioning, I even don’t kiss (greet) my best friend anymore… . These infections spread via air, personal contact” (female, 46 years).
Regarding ease of use, some respondents were bothered by the frequency of administration, administration in hospital, and the intravenous administration. However, they much preferred receiving eculizumab than the dialysis they had received or would otherwise need. While side effects and administration mode were important to respondents, they were extremely grateful and satisfied with the therapy as they felt they could not have survived or participate in everyday life without the medication.
“Without eculizumab I would be on dialysis 3 times a week, with all associated consequences. I wouldn’t be able to participate in society, and I am so young” (female, 24 years).

