For many thousands of aHUS patients around the world just getting access to eculizumab is the most pressing issue; but, increasingly, for many aHUS patients, who have had access to it, the question is becoming about whether it is possible to withdraw treatment, and what is likely to happen if it was stopped.
The Rare Disease Day video ( which has now reached over 600 views) featured such questions, including some from patients who have already been withdrawn from treatment.

Isabella asks “I’ve had aHUS for 2 years now without eculizumab and THANKFULLY without a relapse. Is there any PILL form of eculizumab being worked on so my mommy will feel better about letting me start it? (That way she can stop worrying everyday about what might trigger a relapse)”
Isabella has not had another episode of aHUS for two years; being in remission, whilst a good thing and what aHUS patients would desire most , can also be scary because the original experience of aHUS can leave a lasting feeling of anxiety ( see previous news blog about the psychological impact of aHUS here and pill form of eculizumab here)
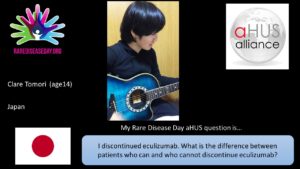
Clare of Japan asks “I discontinued eculizumab. What is the difference between patients who can and who cannot discontinue eculizumab? “
The answer to that Clare will have a significant impact on the future treatment strategy for all aHUS patients as well as how affordable eculizumab can be for the average aHUS patient treated in the future.
In the past few years more and more research is taking place to seek answers to that question .
At its first meeting in Barcelona, the alliance heard from the patient organisation in Italy about the work that had begun on eculizumab dosage adjustment and withdrawal on some Italian patients . Researcher Dr Gianluigi Ardissino has published the results of the Milan Study (click here ); and now has even more evidence from a small group of aHUS covering “many months” from treatment withdrawal; including how many have relapsed with aHUS , as well as what happened to them when treatment was reinstated. The Milan Study also involves how best to alleviate the anxiety through some self monitoring arrangements, and having a safe and sustainable care pathway for those in post treatment remission.
Researchers in France have begun a study on a much bigger scale to see what happens when treatment is withdrawn and what it is about the patients ( in Doctor speak their “genotype”) who relapse or not, to develop a decision “algorythm” . The study called “STOPECU” led by Prof. Fadi Fakouri of Nantes University Hospital is still recruiting in 31 hospitals across France. 60 patients are expected to be involved. More details about it can be read here.
A four year study has also begun in the The Netherlands as part of a recent agreement to extend an existing health policy for access to eculizumab for all aHUS patients. Key hospitals in the Netherlands are participating with Radboud University and lead clinician Dr Nicole van de Kar. aHUS patient organisation aHUS NL are closely involved with that study, which is being called CUREiHUS. More information here
A similar study, led by Prof. Neil Sheerin, is about to begin in the UK as part of the decision by its health institutions NICE and NHS England . A video about the dialogue which has been begun to keep potential participants informed can be seen by clicking here.
Alexion is also undertaking a study of those who have ,or are yet to withdraw from treatment over the next five years. Over 300 aHUS patients are expected to be enrolled in this long term study, which is to be called the “EVIDENCE” Study . Recruitment has commenced at 12 hospitals in Australia and 6 in the USA. Click here for more information
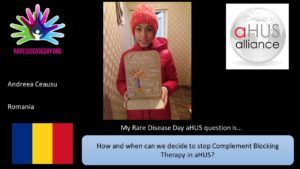
So there is considerable work going to find an answer to Andreea’s question “How and when can we decide to stop Complement Blocking Therapy in aHUS?”
A key word in Andreea’s question is “WE” because the decision does need the patient’s involvement with informed counselling based on best medical evidence about the risks and probabilities of recurrence, and how safe the patient will be without further treatment.
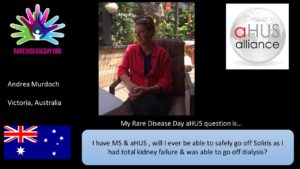
Another Andrea but this time of Victoria Australia asks “I have MS and aHUS so will I ever be able to safely go off eculizumab as I had total kidney failure and with it I was able to go off dialysis?”
This question illustrates even more how individualised the evidence may need to be, to be able to make a clinically safe decisions about treatment withdrawal for each patient.
With the considerable potential evidence from the five studies listed above, along with numerous other published case studies based on an individual, or a small handful of aHUS patients , collectively data will drive treatment strategies and decisions about who will need continual life long treatment, who might only need intermittent treatment as and when needed, or those in full remission whom after the initial treatment of the acute episode will have no further need of eculizumab.
A strategy for provision of eculizumab when patients need it for as long as they need, not only reduces waste of a precious resource, but makes it a more affordable treatment for more aHUS patients who need it, but cannot access it at present.
Such then is the critical importance of getting answers to Isabella, Clare, Andreea and Andrea’s questions.

