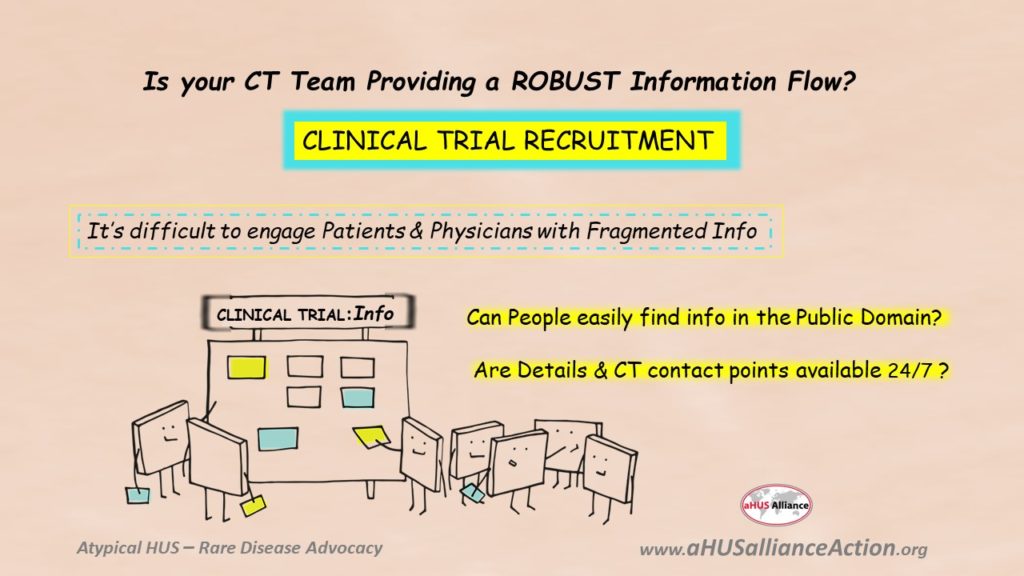
It’s been quite an unsettling end to the first half of 2022 within the atypical HUS community, as July 2022 has begun with an amalgam of issues centered on aHUS information flow. Or more aptly put, people and groups dissatisfied with the fragmented information flow within the aHUS space. This is particularly true regarding clinical trials for people with atypical HUS. While the aHUS Alliance Global Action team has fielded phone calls and emails from not only atypical patients and family caregivers, some might be surprised that physicians also contact our group for assistance. Simply put, information about aHUS clinical trials is scarce and what little is in the public space is disjointed.
Atypical HUS affects only a handful of people per million, which does affect patient numbers regarding clinical trial recruitment. But there are many faects which impede engagement and information flow to patients, clinicians, and investigators. Variations in aHUS nomenclature doesn’t help, nor does the fact that ‘Key Words’ for publications involving new aHUS knowledge and studies may be listed generally under ‘complement’ or ‘thrombotic microangiopathy’ rather than specifically noting atypical HUS. Research groups and pharma often must segregate information and outreach to be compliant within the regulatory environments, and modify information flow according to national policies. Yes, we’d all like to bring new aHUS drugs to patients which are safe and effective but certain barriers remain in place (and often for valid and ongoing reasons).
It’s distressing for our group of international aHUS advocates, an all volunteer group of patients and family caregivers, to receive contact from people or companies who so clearly do not understand the disease nor its impact. People in professional fields of marketing and communications often haven’t done any checking into some very basic, underlying aspects of atypical HUS. “We’d like to offer you the opportunity to provide the names of 3 to 5 patients we can survey for our project.” How will that inform your company of aHUS needs and issues, given that every case of atypical HUS is quite different in terms of disease duration, organs affected, and severity? Their answer: “We’ve worked in the rare disease space before, and are quite comfortable with our methods.” It doesn’t seem to matter to such individuals when we point out that their survey responses will be quite differ from aHUS transplant patients compared to those in nations where aHUS drugs are not available. Compared to other rare disease populations, aHUS is not like the others and some companies circumvent their own efforts by trying to fit a round peg into a square hole.
Perhaps it seems peculiar that we keep reiterating a basic concept taught in Business 101: Understand the Marketplace and Know your Customer. If you’re associated with a research team, pharmaceutical company, or university we’ve likely connected and offered insights into what might be productive for your efforts. If you’re a patient or caregiver, we’ve posted a deep and comprehensive set of articles and resources specific to atypical HUS. If you’re wondering how about the impact and issues of national policies and rare disease groups affect aHUS patients, we’re pleased to report that’s fully discussed within a published article. If you’re wondering about whether new efforts are underway regarding aHUS advocacy in 2022, we’ve got that covered with the launch of our global aHUS Community Advisory Board. Yes, we too don’t understand why aHUS clinical trial information is so scattered and missing important elements which patients and physicians are clamoring to receive. It’s going to be difficult to recruit patients for aHUS clinical trials when neither patients nor their medical teams are able to easily access information. If clinical trial leads and research teams are reaching out to aHUS advocacy groups and to clinicians, have they asked what’s needed? If so, have they listened to and understood concerns and existing barriers, then taken actions with a holistic approach to remedy them?
Excited about reading this? Actually the aHUS Alliance Global Action team has already addressed all of the above, in email and Zoom calls as well as in published documents, website articles, reports from our global polls, and more. Below are a few articles that touch upon points raised here, we hope that reading them will provide background context.
Wishing for more information or faster, better results isn’t enough – individuals and groups must work collaboratively to improve them. We’d like to offer more information about clinical trials and studies, but can only add visibility to what research teams and companies put in the public domain. People first need to gain knowledge and understand insights from different perspectives, but then utilize that background information to create meaningful efforts that move matters forward. Listen and fully comprehend first, then take action.

Gain Context with this sampling of Article Topics
The complex aHUS Arena – Experiencing aHUS: Variations & Viewpoints
Why the aHUS Space is different – Atypical HUS: Not like the Others
Launch of an NFK project group – aHUS Nomenclature
aHUS Advocacy & Patient Centricity – Punching above its aHUS Weight
Community Advisory Board – Atypical HUS CAB
Patient Engagement: Time to re-Define Patient Engagement
Improvement still needed in 2022: aHUS Clinical Trial Barriers
Patient Reported Outcomes: Pros of aHUS
See the “Patients’ Perspective” section, on the effect in the aHUS community regarding Policy & Regulation. Raina et al. Optimal management of aHUS: challenges and solutions