A trial has been designed by Alexion to examine the efficacy of Ravulizumab to treat those with a Thrombotic Microngiopathy , TMA, caused by a trigger. This somewhat vague scoping trial title is needed because it is intended to test efficacy on diseases which are not regarded as aHUS, where Ravulizumab works, nor TTP or HUS where it does n’t .
Nor on postpartum pregnancy aHUS nor haematopoietic stem cell bone marrow transplants TMA , DGKE Syndrome and so on. It’s the exclusions that define the inclusion. Lupus (SLE) or even Antiphospholipid Syndrome (APS)
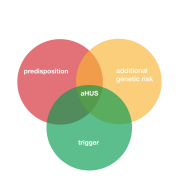
There are TMAs which result from unregulated Complement , even if only temporary hampered by a secondary cause of TMA, a “trigger”. But TMA in these conditions is not fully understood and more evidence is needed.
If it’s not a complement mediated TMA then Ravulizumab will not work because what ever is damaging the patient’s endothelium will still do so and the TMA continues. If it is Complement, but temporary in nature, it might have stopped anyway without an inhibitor.
A really tricky trial to conduct. But if Ravulizumab can be found to work then a whole cohort of patients with an unmet clinically effective treatment need will benefit. As aHUS patients have done.
Full details of the trial can be found at this link to Clinicaltraial.gov NCT04743804
It is a global trial with no planned hospital locations shown. 100 participants will be sought , half of whom will receive Ravulizumab and the other half a placebo. Recruitment has not begun yet. But the intended end date is October 2022, or when sufficient candidates have been monitored for 26 weeks.
This is a topic is of interest like another trial in The Netherlands which featured in a Global Action blog earlier this year “Diagnostic and Risk Criteria for Complement Defects in Thrombotic Microangiopathy and Amplifying Conditions, Such as Severe Hypertension: The COMPETE Study. The blog article itself ( read here) was about the renaming of diseases with TMA links to Complement including the eradication of the term aHUS itself.
Looking around the websites of overlapping diseases with aHUS it is evident that it is only the aHUS patient advocacy community that will be featuring research like these about TMA,
Even if it is not strictly about aHUS as is. But about “new aHUS” as might be.
Including such minority patients from a more common disease communities in a smaller community that so far advocates for them.
Article No. 474