Nearly 400,000 people live in Iceland. At normal prevalence levels there should be two aHUS patients.
There are at least three in Iceland.
They are all in one family. The family is extensive with 203 members living. Five generations involved, at least.
The genetic mutation which predisposes them to aHUS is found in Complement Factor B. It results in a gain of function of the Complement factor which leads to excessive uncontrollable complement activation.
In 20 family members tested, along with three patients who have onset with the disease there are four family members susceptible to , but not affected by aHUS. There is incomplete penetrance of the disease in this family.
The full article about the study of this family by Diana Karpman’s Group can be read HERE. It is published in Kidney International Reports and it is a relatively easy read.
The genetic mutation found in the family was one of the gain of function mutations D371G , full title C1112A> G , p.Asp371 Gly, It was not found in any other family of the nearly 44,000 Icelanders who have had whole genome sequencing for whom data is available.
This Icelandic family gives researchers the opportunity to study that question about why some family members on set and other don’t. In this family two members are identical twins but only one of them has had aHUS so even a bigger opportunity.
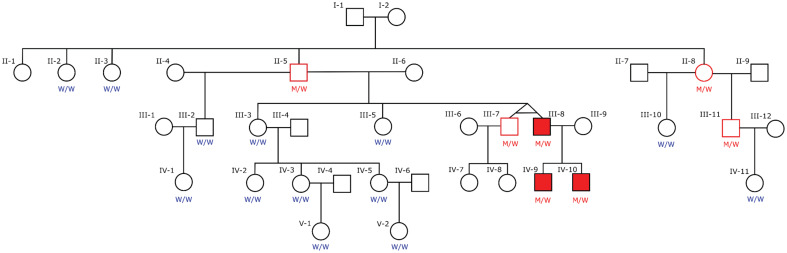
The twins inherited the mutation from their father who was an unaffected carrier. So was the father’s sister. They had inherited it from one of their parents. One of them likely inherited it from an ancestor living in the 19th century.
The twins were male. The unaffected brother has had two sons both of whom are unaffected but have not been tested for carrying the mutation.
The affected brother, who onset with aHUS twenty years ago , has two sons both of whom have had aHUS and tested positive for the mutation.
All three patients are in remission. Does that say something about the risks of CFB mutations?
Blood tests of the twins have shown no significant difference between them for genetic variants, blood Complement levels.
“Most probably (different disease experience by the twins) result from epigenetic variations which may also be linked to environmental exposures such as prenatal and postnatal events, infections, inflammations, vaccinations, as well as lifestyle variations, including body mass index discordance, that may contribute to complement.” was the conclusion of the study group.
The hit that is needed to trigger aHUS in those genetically susceptible
Article No. 574.