Complement inhibition treatment discontinuation is a topic that Global Action has led on in the aHUS patient advocacy world for several years.
More than anyone else our website has provided patients with plenty of evidence to empower them to make an informed decision about stopping treatment. Or not.
It is after all something of importance to us too , as we also are impacted by such decisions.
Knowledgeable empowered patients is our aim.
The following list of eighteen articles demonstrate the build up in knowledge until Global Action proposed a simple decision making model for patients to use in conversations with their doctor about treatment discontinuation ( article 16 in this list).
1 Can eculizumab be stopped? (18 March 2017)
This is the first article in our website’s first year and it was written in response to a question asked in the Rare Disease Day video of 2017. It was the first opportunity to mention the clinical studies on discontinuation which were happening at the time. Including one by Alexion, which was subsequently abandoned.
2. RISK OF WITHDRAWAL OF ECULIZUMAB (30 May 2017)
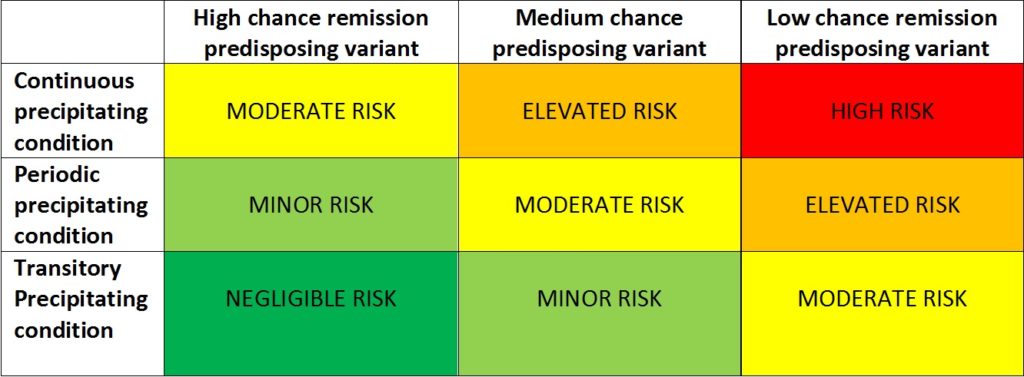
Another reminder of the trials taking place in France The Netherlands and UK. Also an introduction by Global Action to its risk of relapse/ chance of remission matrix, employing predictive triggering and genetic factors to evaluate risk.There are no certainties just degrees of risks.

3. aHUS Trials Watch 5
An update on STOPECU ( NCT02574403) -the French Study of the withdrawal from eculizumab treatment which began in late 2015. ( click here for trial details ) and has planned to…
CONTINUE READING
An article about the design and objectives of the French national trial of eculizumab withdrawal.
4 Remission from aHUS ( 23 April 2019)
A retrospective analysis of aHUS patients who had stopped treatment at University College London Hospital in the UK. Most patients remained in remission and for those that didn’t, some evidence of their particular genetic predisposition to relapse was gleaned. Future discontinuation was not discounted for those who had relapsed.
5 Eculizumab withdrawal risk profiling (4 December 2020)
The first literature research on the topic of discontinuation by the Acosta group presented at ASH20 and which collected evidence of 70% or so of aHUS patients remaining in remission after treatment discontinuation . The Global Action Risk Model was included once more in the website article.
6 “StopEcu” – the French Study reports ( 4 December 2020)
At the same time the results of the French “Stopecu” study were published. 13/55 patients relapsed. Full phenotype and genotype data of predictive factors were given. Some protocols were discussed on how to do it safely. No evidence of an algorithm for withdrawal decision making being developed at this point.
7 Withdrawal evidence patients need to know ( 5 December 2020)
Continuing the flurry of reports on treatment discontinuation the John Hopkins University Hospital team reported their observations in a retrospective review of patients who had discontinued treatment. Providing more conclusions about predictive factors and safe protocols.
8 Eculizumab withdrawal- safe and reasonable? (20 December 2020)
And before the month was out another clinical trial report (interim) from The Netherlands landed providing more evidence about predictive factors and the safe monitoring for relapse and care pathways.
9 Decision Process: Discontinuing aHUS Treatment (18 June 2021)
A Global Action article demonstrating the enormity of information available on treatment withdrawal and the complex maze to be navigated by the patient and doctors to find it to appraise themselves and make an informed decision. Difficulty of getting informed becomes an aspect of the decision making process.
10 Withdrawal – need for a consensus (8 September 2021)
A report from the Alexion Registry added further knowledge to issue of treatment withdrawal. By now Global Action acknowledged there was no paucity of information but nothing was collated nor was a consensus formed. It was established that successful treatment withdrawal was possible, but as far as who it would be and how to do it safely more evidence was needed. Global Action recalled that the Stopecu study in France had an objective to produce a decision making algorithm to predict a withdrawal outcome. At this point Global Action thought one was very much needed.
11 aHUS remission chance and relapse risk (30 March 2022)
The question about the chance of remission was asked by increasing number of patients in the Rare Disease Day video five years on from the first video. Global Action answered all with what was known at that point in time. It was evident that more and more patients wanted to know if they could cease treatment. Again the Global Action’s aHUS remisssion and relapse risk matrix was shown in the article.
12. Monitoring after Eculizumab withdrawal ( 4 April 2022)
Another question from the Rare Disease Day video about treatment discontinuation but this time about a safe withdrawal protocol. Doing it safely to cause no harm is becoming a more important risk matter than the chances of relapse. Best practice guidelines are called for. Patients must be thoroughly informed before consenting to do so.
13 aHUS – Stopping eculizumab treatment safely (1 November 2023)
A report on how the UK’s Stopping Eculizumab Treatment Safely SETS clinical trial has been set up to explore a safe way to withdraw from treatment with an assured return to treatment pathway. Further emphasising the way to avoid harm from the chance of relapse.
14 Stopping Treatment – aHUS Agenda Topic 7 ( 10 December 2023)
A current answer status to the patients research agenda question about discontinuing treatment successfully and were the differences in patients known is “Yes” and “Yes” But a concern about how safe is the way it is done universally remains an area of concern for patients.
15. TO STOP OR NOT TO STOP – THAT IS THE QUESTION? (16 July 2024 )
As there was no consensus emerging and the StopEcu did not produce an algorithm for a decision process, Global Action took it upon itself to make the case for a proposed model for treatment discontinuation decision making by patients. Global Action brought all the evidence from the above reports and much more to create such a model.
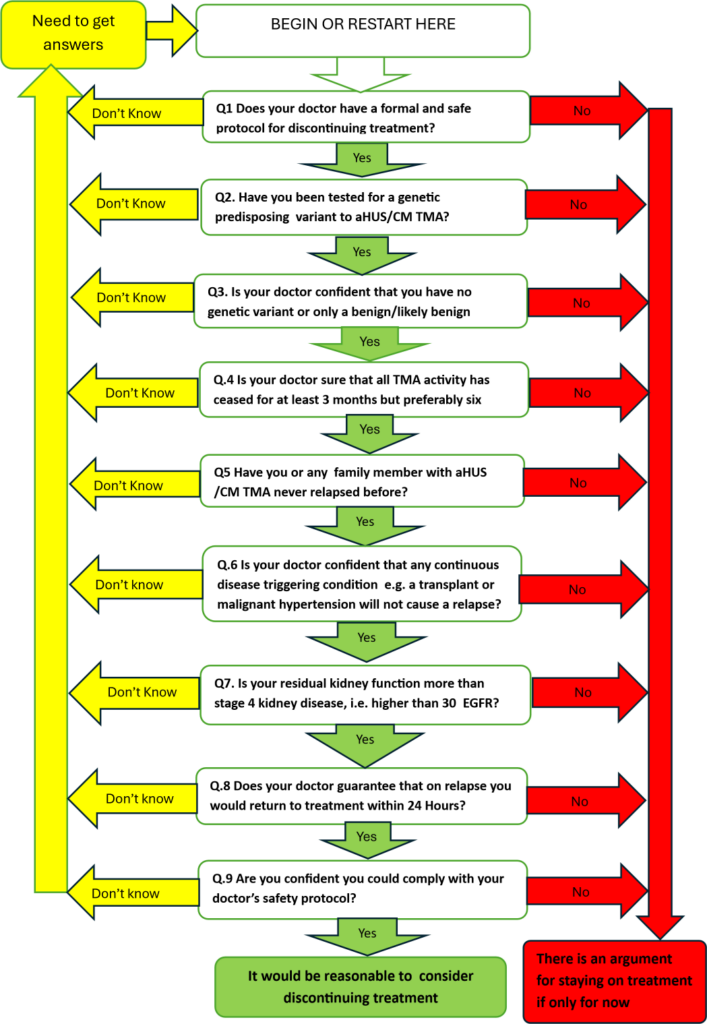
16.AN aHUS PATIENT’S TREATMENT DISCONTINUATION MODEL (16 July 2024)
Global Action published its model ( see below) with full explanation of its use. The aHUS patient community was asked for ways in which the model could be improved.
17 Discontinuing complement inhibitor treatment ( 22 October 2024)
After three months following the proposed model’s publication and promotion not one suggestion as been made on how the decision model for treatment discontinuation can be improved. Not one patient organisation advocate nor a single patient or carer has found anything to change or add.
That is quite remarkable.
18 Treatment Discontinuation- do doctors and patients think alike?
Recently a novel article about complement inhibitor treatment discontinuation was published. Novel because of the research method used to produce its results. A qualitative study based on interviews with ten experts…
This more recent article which shows that doctors’ perceptions and patients’ perceptions on the matter of discontinuation are beginning to converge. Both groups have its critics and both have its supporters.
Global Action’s balanced cautious approach in the decision making model it created is an attempt to define where there might be consensus on this important decision
Does Global Action believe that it going to be adopted universally?
It has been around global patient advocacy a long time so knows there are limitations in advocacy reach and influence.
But even if today it is the best advice about treatment discontinuation available for those patients who need it, including its remission/relapse risk matrix* , Global Action can do no more for patients.
Something else is needed too.
Article No. 709
*latest on the “relapse” version
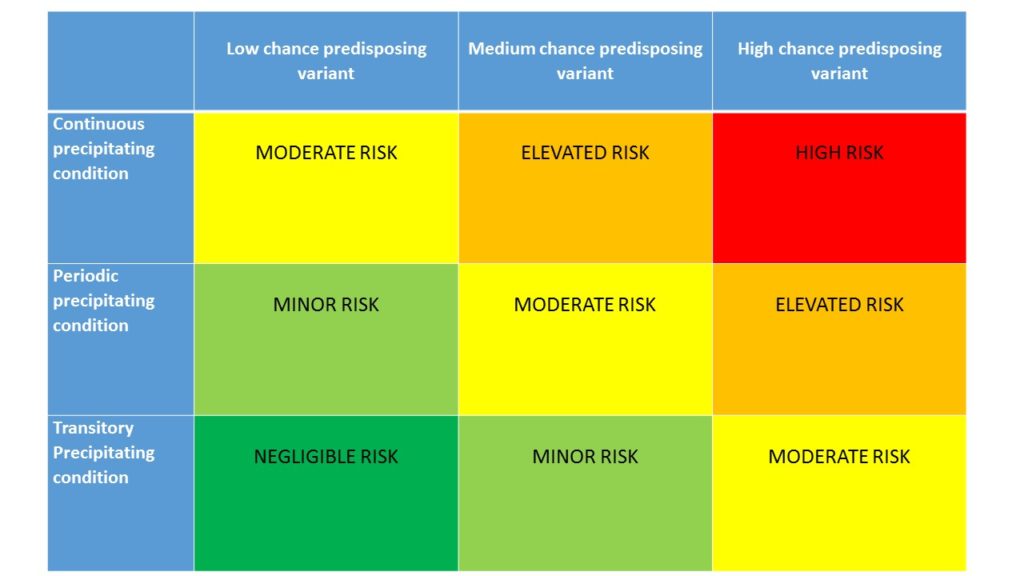
HIGH RISK – 90 to 99.9% risk of relapse
ELEVATED RISK– 70 to 89% risk of relapse
MODERATE RISK– 30 to 69% risk of relapse
MINOR RISK – 10 to 29% risk of relapse
NEGLIGIBLE RISK – 0.1 to 9% risk of relapse
Assuming a “normal” distribution of risk.

