
WWW.AHUSALLIANCEGLOBALACTION.ORG
18 March 2023
aHUS PATIENTS’ VIEWS ON THE ATTRIBUTION OF ADVERSE EVENTS /SIDE EFFECTS EXPERIENCED DUE TO EITHER THEIR PRIMARY DISEASE OR ITS TREATMENT
Amber, Amelie, Amy, Anne-Kathrin, Arthur, Ashley G, Ashley T, Ashlyn, Bethany, Caitlin, Cariann, Carsten, Ciara, Cyrus, Danielle, Darcey , Debbie, Donna, Dorota, Erica, Heather, Emilio, Esmée, Ethan, Isabella, Jace, Jackie, Joana, Kamiryn, Karina, Khloe, Kyle, Lisa M, Lisa S, Michael C, Michael E, Mikaela, Pamela, Riley, Rylee, Samuel, Stephanie, Taylor, Tomas, Victoria, Jeff and Len
INTRODUCTION
atypical Hemolytic Uremic Syndrome, or aHUS, is a primary disease due to a disorder in an intrinsic part of the innate immune system called Complement. The disease manifests because of dysfunction of control within the alternative pathway of Complement which leads to self-damage to the endothelial cells lining of small blood vessels, or capillaries, of organs which triggers a micro thrombotic event known as Thrombotic Microangiopathy. It particularly affects the kidney.
aHUS can be treated by inhibiting the dysfunctional Complement activation. Eculizumab and ravulizumab are two of several complement inhibitors either approved or in development for the treatment of aHUS. Eculizumab was given marketing approval for aHUS use by FDA in 2011 and ravulizumab was approved in 2019. Several other countries were to subsequently approve both technologies. Following marketing approval, both technologies have been monitored for Adverse Events (AEs) or Side Effects (SEs) arising during and after stopping treatment .
aHUS patients have identified that research is needed on whether the Adverse Events /Side Effects , AEs/SEs experienced when on or off treatment with a Complement Inhibitor can be distinguished from those temporary or permanent ongoing ailments which follow an initial onset of aHUS. There is little published research on the topic which can provide answers to that question for aHUS patients.
The purpose of this project was to gather some real-world data on AEs/SEs experienced by aHUS patients having had the disease and Complement Inhibitor treatment and provide greater awareness of this aspect of living with disease.
METHOD
To raise awareness of aHUS , aHUS alliance Global Action produces two online videos each year for Rare Disease Day ( 28th /29th February) and aHUS Awareness Day ( 24th September) . Each video has a specific theme and aHUS patients and families are invited to participate. Participation usually involves answering a specific question about aHUS. For the Rare Disease Day 2023 video, or RDD23 video the theme was AEs/SEs from having had aHUS and treatment with a Complement Inhibitor. Participants were asked about their experience of five AEs/SEs. Three which are known to be common ( headaches , nausea and fatigue ), one which is less common (dizziness) and another which is rare (meningitis). They were also given the opportunity to report other AEs/SEs which they had experienced. Their opinion was sought on whether their AEs/SEs were due to the consequence of having had aHUS or due the complement inhibitor treatment.
An explanation of the aims of the RDD23 video and how to participate was given in the aHUS social media and the aHUS alliance Global Action website ( https://www.ahusallianceaction.org/ahus-community-project-for-rare-disease-day-2023/) on January 8th 2023 and entries were closed on 17th February 2023. The video was released on YouTube on February 28th 2023 and can be viewed at this link HERE. The data provided by participants has been summarised, analysed and is now reported.
RESULTS
There were 44 participants in the RDD23 video. Twenty-nine were adults and 15 children. The genders, based on photographs provided of the participants, were 33 female and 11 males. 27 of the respondents were from the USA and 17 from 10 countries in the rest of the world (Australia, Brazil, Canada, Czech Republic, Faroe Isles, Germany , Hong Kong, Poland , Portugal, UK).
All participants reported that they know how to report an AE/SE.
In Table 1 the AEs/SEs reported by children, adults and all are presented. In total 199 AEs/SEs were reported by participants, 4.5 AEs/SEs per patient on average. Patients attributed 102.5 of them to aHUS and 96.5 to using the Complement Inhibitor. Adults reported the most AEs/SEs 159 and attributed them equally between aHUS and the Complement Inhibitor. Children attributed reported more AEs/SEs to aHUS than the Complement Inhibitor but overall there was little difference between the two potential causes. Adults experienced relatively more AEs/SEs than children, adults averaged 5. 4 per patient and children 2.6, a ratio of just more than 2:1.
Unsurprisingly AEs/SE relating to the three most common conditions were the highest reported. Fatigue at 55 was the AE/SE most reported , followed by Headaches – 37 and Nausea – 28. The condition thought to be less common, Dizziness, was reported by only 16 patients, mostly adults. None of the participants had reported meningitis, which, given its rarity, would have not been expected to have occurred in a cohort of only 44 patients.
Participants reported 31 miscellaneous AEs/SEs conditions, with a total incidence of 63 attributed almost equally between aHUS and Complement Inhibitor. Notable among the miscellaneous conditions were Memory Loss/Brain Fog which were attributed to aHUS by 10 patients and to Complement Inhibitor by 1 participant. High blood pressure was attributed to aHUS by 5 patients and to the Complement Inhibitor by 2. Eleven participants reported pain in joints and stomach which they all attributed to the Complement Inhibitor. Together these five conditions accounted for almost 50% of the miscellaneous AEs/SEs.
Table 1 Adverse Events/Side Effects Attributed
Children (n=15) Adults (n=29) All (n=44)
| aHUS | CI (1) | Total | aHUS | CI | Total | aHUS | CI | Total | |
| Headache (2) | 3 | 4 | 7 | 13 | 17 | 30 | 16 | 21 | 37 |
| Nausea (3) | 4.5 | 1.5 | 6 | 10.5 | 11.5 | 22 | 15 | 13 | 28 |
| Fatigue (4) | 8 | 5 | 13 | 22.5 | 19.5 | 42 | 30.5 | 24.5 | 55 |
| Dizziness (5) | 0.5 | 0.5 | 1 | 8.5 | 6.5 | 15 | 9 | 7 | 16 |
| Meningitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Miscellaneous (6) | 7 | 6 | 13 | 25 | 25 | 50 | 32 | 31 | 63 |
| Total | 23 | 17 | 40 | 79.5 | 79.5 | 159 | 102.5 | 96.5 | 199 |
* Note 1 – CI is Complement Inhibitor, Note 2 – 8 patients attributed AE/SE to both, 4 were undecided* on attribution , Note 3- 7 patients attributed AE/SE to both, 4 were undecided on attribution Note 4- 17 patients attributed AE/SE to both, 5 were undecided on attribution, Note5 – 1 patient attributed AE/SE to both, 4 undecided on attribution Note 6: 31 separate conditions. *undecided attributions were scored as 0.5 for both causes.
DISCUSSION
Serious life-threatening diseases and/or the technologies approved for their treatment rarely, if at all, result in no long term consequences and AEs/SEs are likely to be experienced. Information about them is collected during clinical trials of health technologies and then monitored in real life following market approval and market access, and in some cases with off label use. See the high-level process map in Figure 1.
Figure 1 The AE/SE Reporting Process for eculizumab and ravulizumab
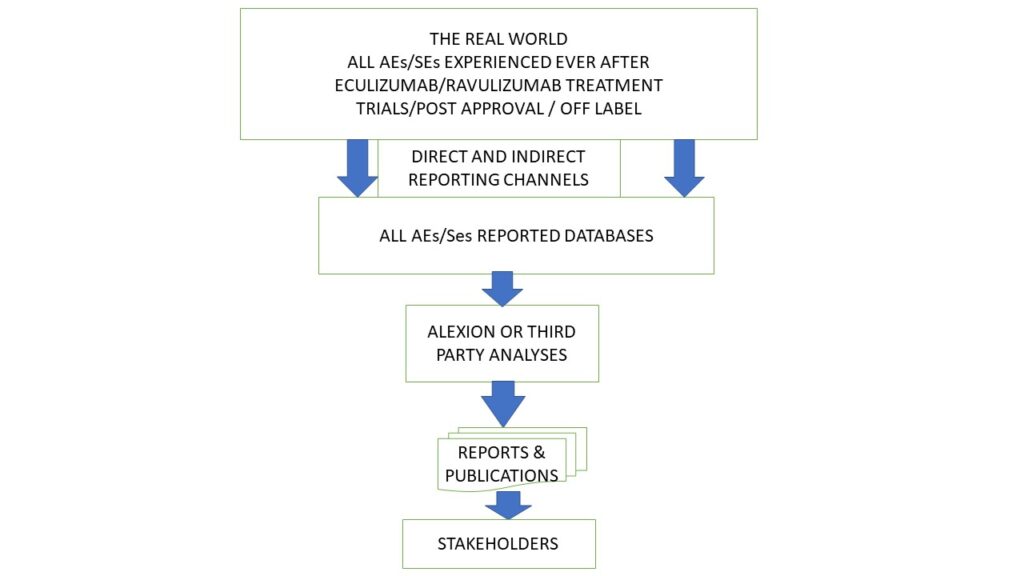
The results of the RDD23 video captured a very small number of real-world events experienced by aHUS patients. The video can be regarded as one of many reporting channels in the AEs/SEs reporting process, as well as being another database and with this article another report to stakeholders.
The patient cohort in the video matches the overall aHUS patient community in several respects. There are more aHUS adults than children, and more females than males, which have been found in other global aHUS studies. The results also match the relative incidence of the selected conditions expressed in the Patient Package Leaflet, which comes with each vial of eculizumab/ravulizumab and which were reported previously by patents in trials.
It differs however from the core AEs/SEs reporting process in two ways. Firstly an attempt has been made to attribute the AEs/SEs to the primary disease aHUS or its treatment. The attributions shown were decided on by the patients who experienced them, rather than by their healthcare professionals. It may be that the latter’s opinions could be different.
Secondly patients, who have not experienced an AEs/ SEs, have been able to report that they have had no experience. The core AEs/SEs reporting process only gathers data from those who have experienced AEs/SEs. As a result, there is no proportionality. To illustrate the point all 44 participants could have potentially reported all of the 31 miscellaneous AEs/SEs, i.e. 1364 possible AEs/SEs but only 63 patients did. Therefore, there are just less than 5% of patients who have experienced these potential AEs/SE. This perhaps illustrates the limited picture that the core AEs/SEs reporting process depicts.
CONCLUSION
A rare disease day awareness event by aHUS alliance Global Action has provided some insight into what has happened to a small cohort of aHUS patients after having experienced the disease and its treatment. Overall, patients have reported that, in their opinion, AEs/SEs are just as likely to be due the long-term effects of the disease as they are to its treatment. Adults are more likely to experience an AE/SE than a child. However potential AEs/SEs have only been experienced by a very small minority of aHUS patients. More research evidence is needed to be able to counsel patients on the longer-term impact of aHUS and how the side effects from treatment could be mitigated.

Article No 570

